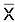 |
关键词:细小病毒H-1;临床安全性;动物,实验;肿瘤
【摘要】 目的 对细小病毒H-1用于临床的安全性作初步评估。方法 本文用Ames试验、哺乳动物细胞染色体畸变试验、小鼠骨髓细胞微核试验、过敏试验和热原试验对H-1用于肿瘤治疗的临床安全性进行了检测。结果 上述所有试验都呈阴性。同时,小鼠对H-1的最大耐受量大于5×1011 空斑形成单位/kg体重,相当于假定的人的临床剂量的2 500倍。对H-1的宿主NB-324K细胞,进行了软琼脂集落试验和裸小鼠体内成瘤试验,其结果也呈阴性。结论 细小病毒H-1对肿瘤治疗是安全的。
A preliminary clinical safety evaluation of parvovirus H-1 on cancer therapy
LIN Wanmin, LING Wenhai, GUO Lanping, et al.Department of Physiology and Biophysics, Fudan University, Shanghai 200433
【Abstract】 Objective To make a preliminary evaluation of parvovirus H-1 for its safety in clinical use.Methods In this study, clinical safety of H-1 in cancer therapy was examined using the Ames test, the mammalian cell chromosome aberration test, the micronucleus calculation of mouse bone marrow cells, the allergy test and the pyrogenicity test.Results Negative results were observed in all the tests. The mouse tolerance test showed that the tolerance limit was at least 5×1011 plaque -forming units/kg weight, which was 2500 times higher than the supposed clinical dose. The H-1 host NB-324K cells were also tested using the anchorage-independent assay and the tumour formation in nude mice. Both of the assays gave negative results. Conclusion All these results preliminarily showed that the H-1 was safe in cancer therapy.
......
您现在查看是摘要页,全文长 25811 字符。