血小板源性伤口愈合因子对伤口组织细胞增殖作用的实验研究
作者:张 艳* 陈荣德* 朱旭东*
单位:* 第三军医大学附属大坪医院野战外科研究所一室(重庆,400042)
关键词:血小板源性伤口愈合因子;成纤维细胞;表皮细胞;增殖
中国修复重建外科杂志980403 摘 要 为了观察不同浓度的血小板源性伤口愈合因子(PDWHF)对伤口成纤维细胞及表皮细胞增殖能力的影响,并探讨其可能的机理,利用离体培养的鼠伤口成纤维细胞及人伤口表皮细胞为模型,将培养细胞分为三组,空白对照组:基质处理组(BM,成分为1640+0.5% FCS);阳性对照组:1640+10%FCS;PDWHF组:BM+1%PDWHF、BM+3%PDWHF、BM+5%PDWHF、BM+7%PDWHF、BM+10%PDWHF及BM+12%PDWHF。待测样本与细胞共孵育48小时后,MTT法测定细胞的增殖情况。结果表明,PDWHF的浓度为1%~7%时,可显著促进伤口成纤维细胞及表皮细胞的增殖,明显高于0.5%FCS组(P<0.01);PDWHF的浓度为10%时,其促增殖作用已不再明显,与0.5%FCS组无显著差异(P>0.05);PDWHF的浓度为12%时,其只对伤口成纤维细胞的增殖呈现出明显抑制作用。认为,PDWHF是多种生长因子的混合物,在一定的浓度范围内对伤口成纤维细胞及表皮细胞有明显的促增殖作用,但当其浓度过高时促增殖作用不明显,甚至有抑制作用。
, 百拇医药
EXPERIMENTAL STUDY OF PROLIFERATION EFFECTS OF PLATELET DERIVED WOUND HEALING FACTOR ON TISSUE CELLS OF WOUND/Zhang Yan, Chen Rongde, Zhu Xudong. Research Institute of Surgery, Daping Hospital, Third Military Medical University, Chongqing, P.R.China 400042
Abstract This experiment was designed to observe the proliferative effects of platelet derived wound healing factor (PDWHF) of different concentrations on fibroblasts from rat wounds and on epithelial from human wounds. Cultured fibroblasts from rat wound and epithelia from human wound were randomly divided into three groups. (1) In blank control, the cells were treated with basic medium (BM, contains 1640/0.5% FCS); (2) the positive control, the cells were treated with 1640/10% FCS and (3)in the PDWHF group, the cells were treated respectively with BM/1% PDWHF, BM/3% PDWHF, BM/5% PDWHF, BM/7% PDWHF, BM/10% PDWHF, BM/12% PDWHF, respectively. The Cells were collected after 48 hours culturing with BM or PDWHF, and the cell proliferation was measured by MTT method according to the OD values. The result showed that the PDWHF could remarkably enhance the proliferation of fibroblasts and epithelial cells when its concentration was between 1% and 7%, which was obviously higher than that of the blank control (P<0.01). When the concentration of PDWHF reached 10%, its proliferative effect was not remarkable when compared with the blank control, When the concentration of PDWHF reached 12%, it showed inhibitory effect on fibroblasts and manifested no obvious inhibitory effect on epithelial cells. It was concluded that the PDWHF was a combination of a variety of growth factors. In a certain range of concentration, the PDWHF might effectively promote the proliferation of fibroblasts and epithelial cells. Howerve, when its concentration reached to relatively higher level, its effect was not remarkable any more, or even showed inhibitory effect on cell proliferation.
, http://www.100md.com
Key words PDWHF Fibroblasts Epithelium Proliferation
导致伤口不愈合的机理十分复杂,至今尚不完全清楚,创面生长因子种类、数量失调是其中的重要环节[1]。外源性生长因子如转化生长因子(transforming growth factors, TGFα/β)、血小板源生长因子(platelet-derived growth factor, PDGF)、a/b成纤维细胞生长因子(a/b fibroblast growth factor, a/bFGF)能明显促进伤口愈合。文献报道,在糖尿病患者难愈性伤口愈合过程中,PDGF、TGFα/β、bFGF、胰岛素样生长因子-Ⅰ、Ⅱ(insulin-like growth factor, IGF-Ⅰ,Ⅱ)、表皮生长因子(epidermal growth factor, EGF)等生长因子的相互配伍使用,可显示出良好的促愈合效果[2~8]。血小板源性伤口愈合生长因子(platelet derived wound healing factor, PDWHF)是来源于血小板脱颗粒时所释放的含多种生长因子的混合提取物,其主要成分为PDGF、IGF-Ⅰ、EGF、TGFα/β等,但对PDWHF促愈合机理的研究国内外尚不多见。我们的实验将重点探讨 PDWHF对伤口不同类型的组织修复细胞的促增殖作用及其可能的机理。
, http://www.100md.com
1 材料与方法
1.1 PDWHF液的制备
取人新鲜血小板浓缩液,以缓冲液充分洗涤后调成浓度为1×109/ml的细胞悬液,加入凝血酶(1 U/ml),离心去除纤维蛋白和残余血小板,上清液经80℃温育30分钟后,再离心除去热变性蛋白,考马斯亮蓝法测定蛋白浓度,制成5 mg/ml的PDWHF应用液,-20℃贮存备用。
1.2 伤口成纤维细胞的培养及传代
按我们建立的方法[9]培养伤口成纤维细胞:随机取体重18 g~20 g的小鼠2只,双侧后肢肌注0.2 ml 1%无菌甲醛液,致伤5天后活杀小鼠。无菌条件下剪取损伤的肌肉组织,经Hank's液和1640培养液(20% FCS、青霉素100 U/ml、链霉素100 μg/ml)各洗涤一次后,剪成大小为0.5 mm3~1 mm3的碎块,加入适量1640液,37℃、5%CO2培养箱中孵育2小时~3小时,组织块贴壁后,以1640培养液洗涤组织块一次,弃掉未贴壁者,加入适量的1640培养液相同条件下培养;7天~9天后成纤维细胞可生长融合成片,期间换液二次。
, 百拇医药
生长融合的细胞经0.25%胰蛋白酶消化30秒,倾去消化液,加入1640液洗涤二次,用1640培养液将细胞调成细胞悬液,浓度为1×104/ml,点接细胞悬液100 μl到96孔培养皿中,37℃、5%CO2条件下孵育12小时,吸去培养液,按加入的孵育液不同,将培养细胞分为三组,空白对照组:基质(basic medium, BM)处理组,成分为1640+0.5%FCS;阳性对照组:1640+10%FCS;PDWHF组:BM+1%PDWHF、BM+3%PDWHF、BM+5%PDWHF、BM+7%PDWHF、BM+10%PDWHF及BM+12%PDWHF。
1.3 表皮细胞的分离、培养及传代
门诊手术切取的包皮去除血污,生理盐水冲洗,清除皮下组织,去真皮和皮下疏松结缔组织,制成2 cm×2 cm的皮片,浸入消化液(0.05%胰酶+0.01%乙二胺四乙酸二钠,1∶1),4℃过夜。剥离表皮层,放入培养液DEME/F12(两种培养液均为Sigma产品,3∶1混合,双蒸水溶解后,加10%FCS,青霉素100 U/ml,链霉素100 μg/ml。培养液中加入胰岛素5 μg/ml,氢化可的松0.5 μg/ml,用碳酸氢钠调pH值至7.2~7.4,过滤除菌后置入4℃冰箱备用)。内吹打,胎盘蓝染色,细胞计数,约20万/ml,接种于培养瓶内,37℃,5%CO2孵育,贴壁48小时后,去除未附壁细胞,更换培养液,此后隔日换液,初代亚融合时间为12天~15天。
, http://www.100md.com
细胞长满贴壁后,吸尽培养液。向培养瓶中加入0.1%胰酶和0.02%乙二胺四乙酸二钠,室温下将培养瓶置于倒置显微镜下观察。待细胞回缩、细胞间隙增大后,立即终止消化,加入培养液,以吸管反复吹打瓶壁细胞,使之脱离瓶壁形成细胞悬液。胎盘蓝染色,计数活细胞,调细胞浓度为5×104/ml,实验分组同前。
1.4 细胞增殖的测定
各组待测样本与细胞孵育48小时后,加入10 μl MTT(5 mg/ml),继续培育4小时。再加入100 μl 20% SDS,37℃放置18小时后,在酶标仪(Bio-Rad450型)570 nm波长处测定每孔溶液的光吸收值。每种待测样本平行测定8份样品(n=8),同时设L 929细胞为正常成纤维细胞生长对照组,培育液为1640+10%FCS。以光吸收值大小表示细胞增殖的相对程度。
1.5 统计学处理
, 百拇医药
所得数据以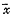 ±s表示,经Microsoft Excel 7.0进行统计分析。P值<0.05为有显著差异,P值<0.01为有非常显著差异。
±s表示,经Microsoft Excel 7.0进行统计分析。P值<0.05为有显著差异,P值<0.01为有非常显著差异。
2 结果
2.1 PDWHF对鼠伤口成纤维细胞增殖的影响
PDWHF在1%~7%的浓度范围内,可显著促进伤口成纤维细胞的增殖,明显高于0.5%FCS组(P<0.01);浓度为10%时,其促增殖作用已不再明显,与0.5%FCS组无显著差异(P>0.05),远远低于10%FCS组(P<0.01)。浓度为12%时,其对伤口成纤维细胞的增殖呈现出明显抑制作用;伤口成纤维细胞在10%FCS培养组与L 929细胞组间无显著差异(P>0.05)。见图1。
2.2 PDWHF对人伤口表皮细胞增殖的影响
, 百拇医药
PDWHF在1%~7%的浓度范围内,可显著促进人伤口表皮细胞的增殖,且明显高于0.5%FCS组(P<0.01);浓度为10%和12%时,其促增殖作用均已不再明显,与0.5%FCS组无显著差异(P>0.05),远远低于10%FCS组(P<0.01),但对人伤口表皮细胞的增殖未见明显的抑制作用(P>0.05)。见图2。
3 讨论
伤口愈合的机理十分复杂,伤口巨噬细胞及伤口微环境中生长因子的种类与数量是
图1 不同浓度的PDWHF对鼠成纤维细胞的增殖作用
图2 不同浓度的PDWHF对人伤口表皮细胞的增殖作用
, 百拇医药
影响伤口愈合过程正常与否的重要因素。文献报道,糖尿病或正常小鼠皮肤中生长因子如PDGF等的含量无差异,但致伤后正常小鼠伤口中PDGF、EGF和a/b FGF等生长因子的峰值水平及mRNA转录明显早于难愈性伤口[10,11]。伤口愈合过程表现为修复细胞的迁移、聚集、增殖、分化、血管再生及胶原合成等。伤口局部各类生长因子间的相互作用无疑是调节伤口组织细胞增殖的重要因素。外源性生长因子如a/bFGF、TGFα/β、EGF等均可明显改善糖尿病所致的难愈性伤口的愈合过程,尤以PDGF、TGFα、FGF等生长因子的配伍使用效果更明显,而EGF必需与胰岛素或IGF-Ⅰ、IGF-Ⅱ配伍使用才能显示促愈合效果[2~10],提示伤口愈合过程中,生长因子的作用是一个多因素相互调节、相互制约的机制。PDWHF作为多种生长因子的天然混合物,其促愈合机理尚不清楚。
我们研究发现,PDWHF浓度为1%~7%时,可刺激低浓度血清培养条件下鼠成纤维细胞及人伤口表皮细胞的增殖。PDWHF中的PDGF、EGF、TGFα是成纤维细胞进入细胞周期的补偿因子,而IGF则是促进因子[12]。虽然TGFβ对体外培养修复细胞生长呈抑制作用[13],但在PDWHF中其抑制细胞增殖作用似乎可被其它生长因子所抑制。当PDWHF浓度为10%时,其促细胞增殖作用已不再明显;浓度达12%时,对成纤维细胞和伤口表皮细胞的影响则有较大的差别。对成纤维细胞的综合作用为抑制效应,对伤口表皮细胞似无明显的作用。由于活体伤口微环境极为复杂,PDWHF对离体培养细胞的影响只能从一个方面反映出在体时其生物学作用的规律[14]。
, http://www.100md.com
PDWHF促进成纤维细胞及伤口表皮细胞的增殖可能与直接刺激细胞促进TGFβ mRNA-Ⅰ型胶原mRNA的表达,进而使细胞合成胶原基质等有关[15,16]。
4 参考文献
1 Ross R. Platelet-derived growth factor. Ann Rev Med, 1987;38:71
2 Westermark B. The molecular and cellular biology of platelet-derived growth factor. Acta Endocrinol Copenh, 1990;123(2):131
3 Hart CE, Bailey M, Curtis DA et al. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimmers in human platelets. Biochem, 1990;29(1):166
, http://www.100md.com
4 Hart CE, Bowen-Pope DF. Platelet-derived growth factor receptor: Current views of the two-subunit model. J Invest Perm, 1990;94(6):535
5 Matsui T, Heidaran M, Miki T et al. Isolation of a novel recpetor DNA establishes the existence of two PDGF recpetor genes. Sci, 1989 Feb 10; 243(4892):800
6 Seifert RA, Hart CE, Phillips PE et al. Two different subunits to create isoform-specific PDGF receptors. J Biol Chem, 1989 May 25;264(15):8771
, 百拇医药
7 Cross M, Dexter TM. Growth factors in development transformation and tumorigenesis. Cell, 1991;64(2):271
8 Pierce GF, Muyyor TA. In vivo incisional wound healing augmented by PDGF and recombinant c-sisgene homodimeric proteins. J Exp Med, 1988;167(3):974
9 Abraham JA, Nhang JL, Tumolo A et al. Human basci fibroblast growth factor: Nucleotide sequence gnomic organization and expression in mammalian cells. Cold Spring Hard. Symp Quant Biol, 1986;51(1):657
, 百拇医药
10 Gospodarowicz D. Fibroblast growth factor: Chemical structure and biology function. Clin Drtho Res, 1990;257(Aug):231
11 Piece GF, Mustoe TA, Lingelbach J et al. Tramsforming growth factor beta reverses the glucocerticoid induced wound healing deficit in rats: Possible regulation in macrophages by PDGF. Pro Natl Acad Sci USA, 1989;86(7):2229
12 Yu W, Naim JO, Lanzafame R et al. Expression of growth factors in early wound healing in rat skin. Surg Med, 1994;15(3):281
, 百拇医药
13 Franzen L, Ghassemifar R, Schultz G et al. Specific binding of EGF in connective tissue repair. Eur J Cell Bid, 1993;60(2):346
14 Grant MB, Khaw PT, Schultz GS et al. Effect of epidermal growth factor, fibroblast growth factor and transfoming growth factor beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci, 1992;33(12):3292
15 Carpen MI. The epidermal growth factor family In: Spom MB, Roberts AB, Editors. Peptide growth factors and their receptors. Berlin Springer Verlag, 1990;31(3):69
16 Bell GI, Fong NM, Stempien MM et al. Human epidermal growth factor precursor cDNA sequence, expression in vitro and gene organization. Nucleic Acid Res, 1986;14(21):8427
(收稿:1997-06-13), 百拇医药
单位:* 第三军医大学附属大坪医院野战外科研究所一室(重庆,400042)
关键词:血小板源性伤口愈合因子;成纤维细胞;表皮细胞;增殖
中国修复重建外科杂志980403 摘 要 为了观察不同浓度的血小板源性伤口愈合因子(PDWHF)对伤口成纤维细胞及表皮细胞增殖能力的影响,并探讨其可能的机理,利用离体培养的鼠伤口成纤维细胞及人伤口表皮细胞为模型,将培养细胞分为三组,空白对照组:基质处理组(BM,成分为1640+0.5% FCS);阳性对照组:1640+10%FCS;PDWHF组:BM+1%PDWHF、BM+3%PDWHF、BM+5%PDWHF、BM+7%PDWHF、BM+10%PDWHF及BM+12%PDWHF。待测样本与细胞共孵育48小时后,MTT法测定细胞的增殖情况。结果表明,PDWHF的浓度为1%~7%时,可显著促进伤口成纤维细胞及表皮细胞的增殖,明显高于0.5%FCS组(P<0.01);PDWHF的浓度为10%时,其促增殖作用已不再明显,与0.5%FCS组无显著差异(P>0.05);PDWHF的浓度为12%时,其只对伤口成纤维细胞的增殖呈现出明显抑制作用。认为,PDWHF是多种生长因子的混合物,在一定的浓度范围内对伤口成纤维细胞及表皮细胞有明显的促增殖作用,但当其浓度过高时促增殖作用不明显,甚至有抑制作用。
, 百拇医药
EXPERIMENTAL STUDY OF PROLIFERATION EFFECTS OF PLATELET DERIVED WOUND HEALING FACTOR ON TISSUE CELLS OF WOUND/Zhang Yan, Chen Rongde, Zhu Xudong. Research Institute of Surgery, Daping Hospital, Third Military Medical University, Chongqing, P.R.China 400042
Abstract This experiment was designed to observe the proliferative effects of platelet derived wound healing factor (PDWHF) of different concentrations on fibroblasts from rat wounds and on epithelial from human wounds. Cultured fibroblasts from rat wound and epithelia from human wound were randomly divided into three groups. (1) In blank control, the cells were treated with basic medium (BM, contains 1640/0.5% FCS); (2) the positive control, the cells were treated with 1640/10% FCS and (3)in the PDWHF group, the cells were treated respectively with BM/1% PDWHF, BM/3% PDWHF, BM/5% PDWHF, BM/7% PDWHF, BM/10% PDWHF, BM/12% PDWHF, respectively. The Cells were collected after 48 hours culturing with BM or PDWHF, and the cell proliferation was measured by MTT method according to the OD values. The result showed that the PDWHF could remarkably enhance the proliferation of fibroblasts and epithelial cells when its concentration was between 1% and 7%, which was obviously higher than that of the blank control (P<0.01). When the concentration of PDWHF reached 10%, its proliferative effect was not remarkable when compared with the blank control, When the concentration of PDWHF reached 12%, it showed inhibitory effect on fibroblasts and manifested no obvious inhibitory effect on epithelial cells. It was concluded that the PDWHF was a combination of a variety of growth factors. In a certain range of concentration, the PDWHF might effectively promote the proliferation of fibroblasts and epithelial cells. Howerve, when its concentration reached to relatively higher level, its effect was not remarkable any more, or even showed inhibitory effect on cell proliferation.
, http://www.100md.com
Key words PDWHF Fibroblasts Epithelium Proliferation
导致伤口不愈合的机理十分复杂,至今尚不完全清楚,创面生长因子种类、数量失调是其中的重要环节[1]。外源性生长因子如转化生长因子(transforming growth factors, TGFα/β)、血小板源生长因子(platelet-derived growth factor, PDGF)、a/b成纤维细胞生长因子(a/b fibroblast growth factor, a/bFGF)能明显促进伤口愈合。文献报道,在糖尿病患者难愈性伤口愈合过程中,PDGF、TGFα/β、bFGF、胰岛素样生长因子-Ⅰ、Ⅱ(insulin-like growth factor, IGF-Ⅰ,Ⅱ)、表皮生长因子(epidermal growth factor, EGF)等生长因子的相互配伍使用,可显示出良好的促愈合效果[2~8]。血小板源性伤口愈合生长因子(platelet derived wound healing factor, PDWHF)是来源于血小板脱颗粒时所释放的含多种生长因子的混合提取物,其主要成分为PDGF、IGF-Ⅰ、EGF、TGFα/β等,但对PDWHF促愈合机理的研究国内外尚不多见。我们的实验将重点探讨 PDWHF对伤口不同类型的组织修复细胞的促增殖作用及其可能的机理。
, http://www.100md.com
1 材料与方法
1.1 PDWHF液的制备
取人新鲜血小板浓缩液,以缓冲液充分洗涤后调成浓度为1×109/ml的细胞悬液,加入凝血酶(1 U/ml),离心去除纤维蛋白和残余血小板,上清液经80℃温育30分钟后,再离心除去热变性蛋白,考马斯亮蓝法测定蛋白浓度,制成5 mg/ml的PDWHF应用液,-20℃贮存备用。
1.2 伤口成纤维细胞的培养及传代
按我们建立的方法[9]培养伤口成纤维细胞:随机取体重18 g~20 g的小鼠2只,双侧后肢肌注0.2 ml 1%无菌甲醛液,致伤5天后活杀小鼠。无菌条件下剪取损伤的肌肉组织,经Hank's液和1640培养液(20% FCS、青霉素100 U/ml、链霉素100 μg/ml)各洗涤一次后,剪成大小为0.5 mm3~1 mm3的碎块,加入适量1640液,37℃、5%CO2培养箱中孵育2小时~3小时,组织块贴壁后,以1640培养液洗涤组织块一次,弃掉未贴壁者,加入适量的1640培养液相同条件下培养;7天~9天后成纤维细胞可生长融合成片,期间换液二次。
, 百拇医药
生长融合的细胞经0.25%胰蛋白酶消化30秒,倾去消化液,加入1640液洗涤二次,用1640培养液将细胞调成细胞悬液,浓度为1×104/ml,点接细胞悬液100 μl到96孔培养皿中,37℃、5%CO2条件下孵育12小时,吸去培养液,按加入的孵育液不同,将培养细胞分为三组,空白对照组:基质(basic medium, BM)处理组,成分为1640+0.5%FCS;阳性对照组:1640+10%FCS;PDWHF组:BM+1%PDWHF、BM+3%PDWHF、BM+5%PDWHF、BM+7%PDWHF、BM+10%PDWHF及BM+12%PDWHF。
1.3 表皮细胞的分离、培养及传代
门诊手术切取的包皮去除血污,生理盐水冲洗,清除皮下组织,去真皮和皮下疏松结缔组织,制成2 cm×2 cm的皮片,浸入消化液(0.05%胰酶+0.01%乙二胺四乙酸二钠,1∶1),4℃过夜。剥离表皮层,放入培养液DEME/F12(两种培养液均为Sigma产品,3∶1混合,双蒸水溶解后,加10%FCS,青霉素100 U/ml,链霉素100 μg/ml。培养液中加入胰岛素5 μg/ml,氢化可的松0.5 μg/ml,用碳酸氢钠调pH值至7.2~7.4,过滤除菌后置入4℃冰箱备用)。内吹打,胎盘蓝染色,细胞计数,约20万/ml,接种于培养瓶内,37℃,5%CO2孵育,贴壁48小时后,去除未附壁细胞,更换培养液,此后隔日换液,初代亚融合时间为12天~15天。
, http://www.100md.com
细胞长满贴壁后,吸尽培养液。向培养瓶中加入0.1%胰酶和0.02%乙二胺四乙酸二钠,室温下将培养瓶置于倒置显微镜下观察。待细胞回缩、细胞间隙增大后,立即终止消化,加入培养液,以吸管反复吹打瓶壁细胞,使之脱离瓶壁形成细胞悬液。胎盘蓝染色,计数活细胞,调细胞浓度为5×104/ml,实验分组同前。
1.4 细胞增殖的测定
各组待测样本与细胞孵育48小时后,加入10 μl MTT(5 mg/ml),继续培育4小时。再加入100 μl 20% SDS,37℃放置18小时后,在酶标仪(Bio-Rad450型)570 nm波长处测定每孔溶液的光吸收值。每种待测样本平行测定8份样品(n=8),同时设L 929细胞为正常成纤维细胞生长对照组,培育液为1640+10%FCS。以光吸收值大小表示细胞增殖的相对程度。
1.5 统计学处理
, 百拇医药
所得数据以
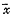 ±s表示,经Microsoft Excel 7.0进行统计分析。P值<0.05为有显著差异,P值<0.01为有非常显著差异。
±s表示,经Microsoft Excel 7.0进行统计分析。P值<0.05为有显著差异,P值<0.01为有非常显著差异。2 结果
2.1 PDWHF对鼠伤口成纤维细胞增殖的影响
PDWHF在1%~7%的浓度范围内,可显著促进伤口成纤维细胞的增殖,明显高于0.5%FCS组(P<0.01);浓度为10%时,其促增殖作用已不再明显,与0.5%FCS组无显著差异(P>0.05),远远低于10%FCS组(P<0.01)。浓度为12%时,其对伤口成纤维细胞的增殖呈现出明显抑制作用;伤口成纤维细胞在10%FCS培养组与L 929细胞组间无显著差异(P>0.05)。见图1。
2.2 PDWHF对人伤口表皮细胞增殖的影响
, 百拇医药
PDWHF在1%~7%的浓度范围内,可显著促进人伤口表皮细胞的增殖,且明显高于0.5%FCS组(P<0.01);浓度为10%和12%时,其促增殖作用均已不再明显,与0.5%FCS组无显著差异(P>0.05),远远低于10%FCS组(P<0.01),但对人伤口表皮细胞的增殖未见明显的抑制作用(P>0.05)。见图2。
3 讨论
伤口愈合的机理十分复杂,伤口巨噬细胞及伤口微环境中生长因子的种类与数量是

图1 不同浓度的PDWHF对鼠成纤维细胞的增殖作用

图2 不同浓度的PDWHF对人伤口表皮细胞的增殖作用
, 百拇医药
影响伤口愈合过程正常与否的重要因素。文献报道,糖尿病或正常小鼠皮肤中生长因子如PDGF等的含量无差异,但致伤后正常小鼠伤口中PDGF、EGF和a/b FGF等生长因子的峰值水平及mRNA转录明显早于难愈性伤口[10,11]。伤口愈合过程表现为修复细胞的迁移、聚集、增殖、分化、血管再生及胶原合成等。伤口局部各类生长因子间的相互作用无疑是调节伤口组织细胞增殖的重要因素。外源性生长因子如a/bFGF、TGFα/β、EGF等均可明显改善糖尿病所致的难愈性伤口的愈合过程,尤以PDGF、TGFα、FGF等生长因子的配伍使用效果更明显,而EGF必需与胰岛素或IGF-Ⅰ、IGF-Ⅱ配伍使用才能显示促愈合效果[2~10],提示伤口愈合过程中,生长因子的作用是一个多因素相互调节、相互制约的机制。PDWHF作为多种生长因子的天然混合物,其促愈合机理尚不清楚。
我们研究发现,PDWHF浓度为1%~7%时,可刺激低浓度血清培养条件下鼠成纤维细胞及人伤口表皮细胞的增殖。PDWHF中的PDGF、EGF、TGFα是成纤维细胞进入细胞周期的补偿因子,而IGF则是促进因子[12]。虽然TGFβ对体外培养修复细胞生长呈抑制作用[13],但在PDWHF中其抑制细胞增殖作用似乎可被其它生长因子所抑制。当PDWHF浓度为10%时,其促细胞增殖作用已不再明显;浓度达12%时,对成纤维细胞和伤口表皮细胞的影响则有较大的差别。对成纤维细胞的综合作用为抑制效应,对伤口表皮细胞似无明显的作用。由于活体伤口微环境极为复杂,PDWHF对离体培养细胞的影响只能从一个方面反映出在体时其生物学作用的规律[14]。
, http://www.100md.com
PDWHF促进成纤维细胞及伤口表皮细胞的增殖可能与直接刺激细胞促进TGFβ mRNA-Ⅰ型胶原mRNA的表达,进而使细胞合成胶原基质等有关[15,16]。
4 参考文献
1 Ross R. Platelet-derived growth factor. Ann Rev Med, 1987;38:71
2 Westermark B. The molecular and cellular biology of platelet-derived growth factor. Acta Endocrinol Copenh, 1990;123(2):131
3 Hart CE, Bailey M, Curtis DA et al. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimmers in human platelets. Biochem, 1990;29(1):166
, http://www.100md.com
4 Hart CE, Bowen-Pope DF. Platelet-derived growth factor receptor: Current views of the two-subunit model. J Invest Perm, 1990;94(6):535
5 Matsui T, Heidaran M, Miki T et al. Isolation of a novel recpetor DNA establishes the existence of two PDGF recpetor genes. Sci, 1989 Feb 10; 243(4892):800
6 Seifert RA, Hart CE, Phillips PE et al. Two different subunits to create isoform-specific PDGF receptors. J Biol Chem, 1989 May 25;264(15):8771
, 百拇医药
7 Cross M, Dexter TM. Growth factors in development transformation and tumorigenesis. Cell, 1991;64(2):271
8 Pierce GF, Muyyor TA. In vivo incisional wound healing augmented by PDGF and recombinant c-sisgene homodimeric proteins. J Exp Med, 1988;167(3):974
9 Abraham JA, Nhang JL, Tumolo A et al. Human basci fibroblast growth factor: Nucleotide sequence gnomic organization and expression in mammalian cells. Cold Spring Hard. Symp Quant Biol, 1986;51(1):657
, 百拇医药
10 Gospodarowicz D. Fibroblast growth factor: Chemical structure and biology function. Clin Drtho Res, 1990;257(Aug):231
11 Piece GF, Mustoe TA, Lingelbach J et al. Tramsforming growth factor beta reverses the glucocerticoid induced wound healing deficit in rats: Possible regulation in macrophages by PDGF. Pro Natl Acad Sci USA, 1989;86(7):2229
12 Yu W, Naim JO, Lanzafame R et al. Expression of growth factors in early wound healing in rat skin. Surg Med, 1994;15(3):281
, 百拇医药
13 Franzen L, Ghassemifar R, Schultz G et al. Specific binding of EGF in connective tissue repair. Eur J Cell Bid, 1993;60(2):346
14 Grant MB, Khaw PT, Schultz GS et al. Effect of epidermal growth factor, fibroblast growth factor and transfoming growth factor beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci, 1992;33(12):3292
15 Carpen MI. The epidermal growth factor family In: Spom MB, Roberts AB, Editors. Peptide growth factors and their receptors. Berlin Springer Verlag, 1990;31(3):69
16 Bell GI, Fong NM, Stempien MM et al. Human epidermal growth factor precursor cDNA sequence, expression in vitro and gene organization. Nucleic Acid Res, 1986;14(21):8427
(收稿:1997-06-13), 百拇医药