叔丁基苯哌嗪取代硫醚类化合物的合成及其抗变态、抗炎活性
作者:王立升 周永红 刘百里 计志忠
单位:王立升 周永红 广西大学工业测试实验中心,南宁 530004;刘百里 计志忠 沈阳药科大学制药系,沈阳 110015
关键词:叔丁基苯哌嗪取代硫醚类衍生物;抗变态;抗炎
中国药物化学杂志 CHINESE JOURNAL OF MEDICINAL CHEMISTRY 1999年 第9卷 第4期 vol摘 要 设计并合成了13个未见文献报道的叔丁基苯哌嗪取代硫醚类目标化合物,并经元素分析、核磁共振氢谱及红外光谱确证其化学结构.对部分目标化合物进行了抗变态和抗炎等生物活性测定.结果表明:W01,W03,W07和W09具有较强的抗变态活性.
Synthesis and Antiallergic,Antiinflammatory activities
, http://www.100md.com
of Substituted t-Butylphenylpiperazinylthio-ether Derivatives
Wang Lisheng,Zhou Yonghong
(Industrial Testing and Experimental Center,Guangxi University,Nanning 530004)
Liu Baili,Ji Zhizhong
(Department of Pharmaceutics,Shenyang Pharmaceutical University,Shengyang 110015)
Abstract Thirteen substituted t-butylphenylpiperazinylthio-ether derivatives were designed and synthesized which were identified by IR,1H-NMR spectral and elemental analysis and had not been found in literature.Some of them were evaluated from the antiallergic and antiinflammatory tests.The results showed that W01,W03,W07and W09 had stronger antiallergic effects.
, http://www.100md.com
Key words substituted t-butylphenylpiperazinylthio-ether derivatives;antiallergy;antiinflammation
1 抗变态反应及抗炎双重活性化合物的设计
哌嗪类抗组胺药物在抗组胺药物中占有较大的比例〔1〕,其抗组胺作用大都强而持久,较少产生嗜睡,属于强效及长效H1受体拮抗剂.分析哌嗪类抗组胺药物的结构,发现大都具有二苯甲基哌嗪母核结构,故认为二苯甲基哌嗪母核为哌嗪类抗变态反应药物的活性必要部分.
Mueller,Richard August〔2〕报道了一系列2,6-二叔丁基酚类衍生物能阻断花生四烯酸的5-脂氧酶代谢途径,阻断白三烯的形成,而白三烯是产生过敏性哮喘的炎症介质,因而该类化合物具有抗变态反应及抗炎双重活性.其中化合物(Ⅰ)和(Ⅱ)作用较强.采用与二苯甲基哌嗪并合原理,设计出13个目标化合物(Ⅲ,W01~W13),其结构和分析数据见表1,2.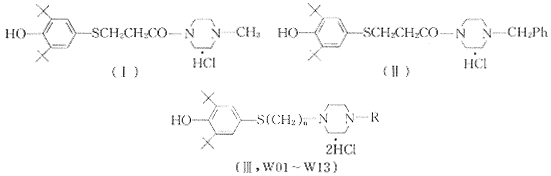
, http://www.100md.com
Tab.1 Structures,physical data of substituted t-butylphenylpiperazinylthio-ether derivatives
Compd.
n
R
Formular
Yield/%
mp/℃
Elemental analysis/%
Calcd.(Found)
C
, 百拇医药
H
N
W01
2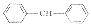
C33H46Cl2N2OS
34.0
115~116
67.22
(67.21
, 百拇医药 7.86
7.92
4.75
5.04)
W02
2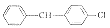
C33H45Cl3N2OS
62.5
140~142
, 百拇医药 63.51
(63.60
7.27
7.30
4.49
4.27)
W03
2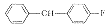
C33H45FCl2N2OS
, 百拇医药 34.6
112~114
65.23
(65.22
7.46
7.50
4.61
4.88)
W04
3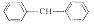
C34H48Cl2N2OS
, 百拇医药
21.6
144~146
67.65
(67.62
8.01
8.07
4.64
4.69)
W05
3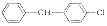
C34H47Cl3N2OS
, 百拇医药
25.1
138~139
64.00
(64.15
7.42
7.57
4.39
4.42)
W06
3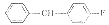
C34H47FCl2N2OS
, 百拇医药
33.8
142~143
65.69
(65.65
7.62
7.68
4.51
4.55)
W07
4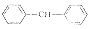
C35H50Cl2N2OS
, 百拇医药
8.1
110~111
68.06
(67.97
8.16
7.80
4.54
4.41)
W08
4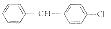
C35H49Cl3N2OS
, 百拇医药
21.9
96~97
64.46
(64.43
7.57
7.67
4.30
4.55)
W09
4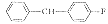
C36H49FCl2N2S
, 百拇医药
18.9
117~119
66.13
(66.19
7.77
7.82
4.41
4.46)
W10
2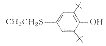
C36H60Cl2N2O2S2
, http://www.100md.com
28.3
215~217
(dec.)
62.87
(62.81
8.79
8.96
4.07
4.17)
W11
3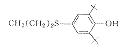
, 百拇医药
C38H64Cl2N2O2S2
32.7
212~213
(dec.)
63.76
(63.69
9.01
9.11
3.91
4.01)
W12
, 百拇医药
4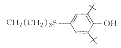
C40H68Cl2N2O2S2
23.6
209~210
(dec.)
64.58
(64.49
9.21
9.40
, 百拇医药
3.77
3.48)
W13
6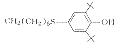
C44H76Cl2N2O2S2
9.8
194~195
(dec.)
66.06
, 百拇医药
(66.10
9.58
9.55
3.50
3.47)
Tab.2 Spectral data of substituted t-butylphenylpiperazinylthio-ether derivatives
Compd.
IR(KBr) /cm-1
/cm-1
, 百拇医药
1H-NMR δ
W01
3618(O—H),2958(CH3∶C—H),2361(N+—H)
1.37(s,18H),2.80(br s,2H),3.20~3.80(m,10H),4.50(s,1H),7.13(s,2H),7.30(m,6H),7.44(br s,4H),10.40(s,1H),2.50(d,H—DMSO-d5)
W02
3616(O—H),2961(CH3∶C—H),2412(N+—H),1493(Ar∶ CC ),1310(C—O),1233(O—H)
, 百拇医药
1.43(s,18H),3.26(br s,4H),3.48(br s,4H),3.94(br s,2H),4.31(br s,2H),5.06(s,1H),5.36(s,1H),7.25(s,2H),7.42(m,5H),7.85(br s,4H),13.60(s,1H),7.26(s,HCCl3)
W03
3620(O—H),2959(CH3∶C—H),2361(N+—H),1606,1511(Ar∶ CC ),1307(C—O),1235(O—H)
1.41(s,18H),3.23(s,4H),3.44(br s,4H),3.89(br s,2H),4.25(br s,2H),5.00(s,1H),5.33(d,1H),7.10(m,2H),7.22(s,2H),7.40(m,3H),7.83(m,4H),13.43(s,1H),13.95(s,1H),7.24(d,HCCl3)
, http://www.100md.com
W04
3619(O—H),2954(CH3∶C—H),2422(N+—H),1307(C—O),1238(O—H)
1.40(s,18H),2.13(m,2H),2.93(t,2H),3.25(brs,2H),3.47(m,4H),4.00(m,2H),4.35(br s,2H),5.09(s,1H),5.27(s,1H),7.21(s,2H),7.40(m,6H),7.88(d,4H),13.35(s,1H),13.97(s,1H),7.26(s,HCCl3)
W05
3618(O—H),2956(CH3∶C—H),2535,2418(N+—H),1489(Ar∶ CC ),1306(C—O),1231(O—H)
, http://www.100md.com
1.42(s,18H),2.17(m,2H),2.85~2.93(m,8H),3.39(m,2H),3.74(br s,2H),4.37(s,1H),5.27(s,1H),7.21(s,2H),7.32(m,5H),7.47(br s,4H),12.94(s,1H),7.27(s,HCCl3)
W06
3619(O—H),2954(CH3∶C—H),2420(N+—H),1307(C—O),1236(O—H)
1.39(s,18H),1.95(m,2H),2.91(t,2H),3.21~4.00(m,10H),5.42(s,1H),7.10(s,2H),7.38(m,5H),7.80(d,4H),11.62(s,1H),2.50(s,H—DMSO-d5)
, http://www.100md.com
W07
3620(O—H),2954(CH3∶C—H),2438(N+—H),1309(C—O),1236(O—H)
1.41(s,18H),1.61(br s,2H),2.00(br s,2H),2.82(t,2H),3.05(br s,2H),3.44(m,4H),3.94(br s,2H),4.35(br s,2H),4.92(s,1H),5.22(s,1H),7.19(s,2H),7.42(m,6H),7.83(br s,4H),13.37(s,1H),13.95(s,1H),7.25(s,HCCl3)
W08
3624(O—H),2956(CH3∶C—H),2436(N+—H),1310(C—O),1234(O—H)
, 百拇医药
1.42(s,18H),1.63(br s,2H),2.00(br s,2H),2.83(t,2H),3.06(br s,2H),3.45(m,4H),3.96(br s,2H),4.32(br s,2H),4.94(s,1H),5.23(s,1H),7.20(s,2H),7.44(m,5H),7.84(br s,4H),13.36(s,1H),13.97(s,1H),7.26(s,HCCl3)
W09
3617(O—H),2958(CH3∶C—H),2428(N+—H),1606,1513(Ar∶ CC ),1308(C—O),1235(O—H)
1.42(s,18H),1.70(m,2H),2.00(s,2H),2.84(t,2H),3.12(br s,2H),3.49(br s,4H),3.95(br s,2H),4.24(br s,2H),5.20(s,1H),5.23(s,1H),7.12(m,2H),7.19(s,2H),7.42(m,3H),7.99(br s,4H),13.23(s,1H),14.24(s,1H)
, http://www.100md.com
W10
3634(O—H),2958(CH3∶C—H),2396(N+—H),1316(C—O),1234(O—H)
1.43(s,36H),3.26(br s,8H),3.60(m,4H),4.00(br s,4H),5.37(s,2H),7.25(s,4H),13.72(s,1H),7.28(s,HCCl3)
W11
3633(O—H),2958(CH3∶C—H),2362(N+—H),1312(C—O),1233(O—H)
1.42(s,36H),2.14(m,4H),2.93(t,4H),3.42(t,4H),3.52(m,4H),4.02(m,4H),5.27(s,2H),7.21(s,4H),3.58(s,1H),7.26(s,HCCl3)
, 百拇医药
W12
3635(O—H),2955(CH3∶C—H),2364(N+—H),1314(C—O),1233(O—H)
1.37(s,36H),1.58(m,4H),1.77(br s,4H),2.87(t,4H),3.11(br s,4H),3.30(br s,4H),3.58(m,4H),7.08(s,4H),11.30(s,1H),2.50(H—DMSO-d5)
W13
3639(O—H),2954(CH3∶C—H),2331(N+—H),1312(C—O),1232(O—H)
1.36(s,36H),1.49~1.68(m,16H),2.83(t,4H),3.08(br s,4H),3.45(br s,4H),3.68(br s,4H),7.07(d,4H),11.62(s,1H),2.50(t,H—DMSO-d5)
, 百拇医药
2 合成实验
熔点用毛细管法测定,温度未经校正.红外光谱采用Bruker IFS 55 型仪器测定,溴化钾压片.核磁共振氢谱采用Bruker AC(E)-250 NMR仪和Bruker ARX-300 NMR仪测定,TMS为内标.元素分析采用Carlo Erba-1106型元素分析仪测定.
2.1 1-〔2-(3,5-二叔丁基-4-羟基苯基)硫〕乙基-4-二苯甲基哌嗪盐酸盐(W01)的制备
参考文献〔5〕制备2,6-二叔丁基-4-巯基苯酚,收率:90.5%,mp 86~88℃.
将2.4 g(0.01 mol)2,6-二叔丁基-4-巯基苯酚加入10 mL1,2-二溴乙烷中,加入2 mL三乙胺及0.1 g碘化钾,室温搅拌8 h,水洗一次,稀盐酸洗,再水洗两次,干燥,蒸出过量1,2-二溴乙烷,残留物备用.
, http://www.100md.com
取2.0 g(0.008 mol)二苯甲基哌嗪加入上述残留物中,加入2 mL三乙胺、0.1 g碘化钾及15 mL甲苯,回流14 h,用盐酸处理后,乙醇-水重结晶,得2.0 g白色晶体,收率:34.0%,mp 115~116℃.
W02~W13参考W01的制备方法制备,其收率及熔点见表1.
3 药理实验
3.1 抗变态反应药理实验结果与讨论
选定DNCB诱导的小鼠接触性皮炎药理模型〔3〕,对8个化合物进行了抗变态活性测定,结果见表3.
Tab.3 Effects of W-compounds on the delayed-type hypersensitivity responses to DNCB in mice
, 百拇医药
Treatment
Dose/mg.kg-1
Animal number
Ear swelling
(x±s)
Inhibition/%
P
control
-
10
33.2±6.1
, http://www.100md.com -
-
hydrocortisone
20×5
10
13.4±8.6
59.7
<0.01
W01
100×5
8
16.4±7.2
48.8
, 百拇医药
<0.01
W02
100×5
8
26.0±6.3
6.9
>0.05
W03
100×5
8
15.4±9.5
53.4
<0.01
, http://www.100md.com
W04
100×5
8
28.5±9.0
10.6
>0.05
W05
100×5
8
29.6±7.7
7.1
>0.05
W06
, http://www.100md.com
100×5
8
27.9±8.3
15.9
>0.05
W07
100×5
7
25.4±9.2
23.3
<0.05
W09
100×5
, 百拇医药
7
14.2±4.9
57.1
<0.01
与空白组对照,其中W01,W03,W07及W09的抑制率具有显著性.W01,W03和W09的抑制率较高,P<0.01,与氢化可的松活性相当.分析其结构,说明在该类化合物中,碳链为两个碳和四个碳时活性较高,并且含有氟原子的化合物活性较强.
3.2 抗炎药理实验结果及讨论
抗炎药理实验选用巴豆油致小鼠耳廓肿胀法〔4〕,结果见表4.Tab.4 Influences on crotonoil-induced ear swelling
Treatment
, 百拇医药
Dose/mg.kg-1
Animal number
Footpad swelling
(x±s)
Inhibition/%
P
control
-
8
24.19±3.44
-
W03
, 百拇医药
150
8
24.06±3.99
0.54
>0.05
W10
150
8
27.55±5.43
0
>0.05
indometacin
20
, 百拇医药
8
16.56±2.82
31.5
<0.01
ibuprofen
100
8
22.69±4.75
6.2
>0.05
抗炎药理实验结果表明W03和W10没有抗炎活性.
作者简介:王立升 通讯联系人
, 百拇医药
参考文献
1 王泽民,康葵,冯梅.当代结构药物全集.北京:北京科学技术出版社,1993.1977~2040
2 Mueller RA.Novel heterocyclic amides.EP 190685.1986-08-13
3 戴岳,杭秉茜,孟庆玉,等.齐墩果酸对变态反应的抑制作用.中国药理学报,1988,9(6):562~565
4 徐叔云,卞如濂,陈修.药理实验方法学.第二版.北京:人民卫生出版社,1994.719
5 Fuzisawa T,Hata K,Kojima T.The sulfurization of sterically hindered phenol with sulfur.A convenient synthesis of 4,4′-thio-bis-〔2,6-dialkylphenols〕and 2,6-dialkyl-4-mercaptophenols.Synthesis,1973,1:38~39
收稿日期:1999-08-09, http://www.100md.com
单位:王立升 周永红 广西大学工业测试实验中心,南宁 530004;刘百里 计志忠 沈阳药科大学制药系,沈阳 110015
关键词:叔丁基苯哌嗪取代硫醚类衍生物;抗变态;抗炎
中国药物化学杂志 CHINESE JOURNAL OF MEDICINAL CHEMISTRY 1999年 第9卷 第4期 vol摘 要 设计并合成了13个未见文献报道的叔丁基苯哌嗪取代硫醚类目标化合物,并经元素分析、核磁共振氢谱及红外光谱确证其化学结构.对部分目标化合物进行了抗变态和抗炎等生物活性测定.结果表明:W01,W03,W07和W09具有较强的抗变态活性.
Synthesis and Antiallergic,Antiinflammatory activities
, http://www.100md.com
of Substituted t-Butylphenylpiperazinylthio-ether Derivatives
Wang Lisheng,Zhou Yonghong
(Industrial Testing and Experimental Center,Guangxi University,Nanning 530004)
Liu Baili,Ji Zhizhong
(Department of Pharmaceutics,Shenyang Pharmaceutical University,Shengyang 110015)
Abstract Thirteen substituted t-butylphenylpiperazinylthio-ether derivatives were designed and synthesized which were identified by IR,1H-NMR spectral and elemental analysis and had not been found in literature.Some of them were evaluated from the antiallergic and antiinflammatory tests.The results showed that W01,W03,W07and W09 had stronger antiallergic effects.
, http://www.100md.com
Key words substituted t-butylphenylpiperazinylthio-ether derivatives;antiallergy;antiinflammation
1 抗变态反应及抗炎双重活性化合物的设计
哌嗪类抗组胺药物在抗组胺药物中占有较大的比例〔1〕,其抗组胺作用大都强而持久,较少产生嗜睡,属于强效及长效H1受体拮抗剂.分析哌嗪类抗组胺药物的结构,发现大都具有二苯甲基哌嗪母核结构,故认为二苯甲基哌嗪母核为哌嗪类抗变态反应药物的活性必要部分.
Mueller,Richard August〔2〕报道了一系列2,6-二叔丁基酚类衍生物能阻断花生四烯酸的5-脂氧酶代谢途径,阻断白三烯的形成,而白三烯是产生过敏性哮喘的炎症介质,因而该类化合物具有抗变态反应及抗炎双重活性.其中化合物(Ⅰ)和(Ⅱ)作用较强.采用与二苯甲基哌嗪并合原理,设计出13个目标化合物(Ⅲ,W01~W13),其结构和分析数据见表1,2.

, http://www.100md.com
Tab.1 Structures,physical data of substituted t-butylphenylpiperazinylthio-ether derivatives
Compd.
n
R
Formular
Yield/%
mp/℃
Elemental analysis/%
Calcd.(Found)
C
, 百拇医药
H
N
W01
2
C33H46Cl2N2OS
34.0
115~116
67.22
(67.21
, 百拇医药 7.86
7.92
4.75
5.04)
W02
2
C33H45Cl3N2OS
62.5
140~142
, 百拇医药 63.51
(63.60
7.27
7.30
4.49
4.27)
W03
2
C33H45FCl2N2OS
, 百拇医药 34.6
112~114
65.23
(65.22
7.46
7.50
4.61
4.88)
W04
3
C34H48Cl2N2OS
, 百拇医药
21.6
144~146
67.65
(67.62
8.01
8.07
4.64
4.69)
W05
3
C34H47Cl3N2OS
, 百拇医药
25.1
138~139
64.00
(64.15
7.42
7.57
4.39
4.42)
W06
3
C34H47FCl2N2OS
, 百拇医药
33.8
142~143
65.69
(65.65
7.62
7.68
4.51
4.55)
W07
4
C35H50Cl2N2OS
, 百拇医药
8.1
110~111
68.06
(67.97
8.16
7.80
4.54
4.41)
W08
4
C35H49Cl3N2OS
, 百拇医药
21.9
96~97
64.46
(64.43
7.57
7.67
4.30
4.55)
W09
4
C36H49FCl2N2S
, 百拇医药
18.9
117~119
66.13
(66.19
7.77
7.82
4.41
4.46)
W10
2
C36H60Cl2N2O2S2
, http://www.100md.com
28.3
215~217
(dec.)
62.87
(62.81
8.79
8.96
4.07
4.17)
W11
3
, 百拇医药
C38H64Cl2N2O2S2
32.7
212~213
(dec.)
63.76
(63.69
9.01
9.11
3.91
4.01)
W12
, 百拇医药
4
C40H68Cl2N2O2S2
23.6
209~210
(dec.)
64.58
(64.49
9.21
9.40
, 百拇医药
3.77
3.48)
W13
6
C44H76Cl2N2O2S2
9.8
194~195
(dec.)
66.06
, 百拇医药
(66.10
9.58
9.55
3.50
3.47)
Tab.2 Spectral data of substituted t-butylphenylpiperazinylthio-ether derivatives
Compd.
IR(KBr)
, 百拇医药
1H-NMR δ
W01
3618(O—H),2958(CH3∶C—H),2361(N+—H)
1.37(s,18H),2.80(br s,2H),3.20~3.80(m,10H),4.50(s,1H),7.13(s,2H),7.30(m,6H),7.44(br s,4H),10.40(s,1H),2.50(d,H—DMSO-d5)
W02
3616(O—H),2961(CH3∶C—H),2412(N+—H),1493(Ar∶ CC ),1310(C—O),1233(O—H)
, 百拇医药
1.43(s,18H),3.26(br s,4H),3.48(br s,4H),3.94(br s,2H),4.31(br s,2H),5.06(s,1H),5.36(s,1H),7.25(s,2H),7.42(m,5H),7.85(br s,4H),13.60(s,1H),7.26(s,HCCl3)
W03
3620(O—H),2959(CH3∶C—H),2361(N+—H),1606,1511(Ar∶ CC ),1307(C—O),1235(O—H)
1.41(s,18H),3.23(s,4H),3.44(br s,4H),3.89(br s,2H),4.25(br s,2H),5.00(s,1H),5.33(d,1H),7.10(m,2H),7.22(s,2H),7.40(m,3H),7.83(m,4H),13.43(s,1H),13.95(s,1H),7.24(d,HCCl3)
, http://www.100md.com
W04
3619(O—H),2954(CH3∶C—H),2422(N+—H),1307(C—O),1238(O—H)
1.40(s,18H),2.13(m,2H),2.93(t,2H),3.25(brs,2H),3.47(m,4H),4.00(m,2H),4.35(br s,2H),5.09(s,1H),5.27(s,1H),7.21(s,2H),7.40(m,6H),7.88(d,4H),13.35(s,1H),13.97(s,1H),7.26(s,HCCl3)
W05
3618(O—H),2956(CH3∶C—H),2535,2418(N+—H),1489(Ar∶ CC ),1306(C—O),1231(O—H)
, http://www.100md.com
1.42(s,18H),2.17(m,2H),2.85~2.93(m,8H),3.39(m,2H),3.74(br s,2H),4.37(s,1H),5.27(s,1H),7.21(s,2H),7.32(m,5H),7.47(br s,4H),12.94(s,1H),7.27(s,HCCl3)
W06
3619(O—H),2954(CH3∶C—H),2420(N+—H),1307(C—O),1236(O—H)
1.39(s,18H),1.95(m,2H),2.91(t,2H),3.21~4.00(m,10H),5.42(s,1H),7.10(s,2H),7.38(m,5H),7.80(d,4H),11.62(s,1H),2.50(s,H—DMSO-d5)
, http://www.100md.com
W07
3620(O—H),2954(CH3∶C—H),2438(N+—H),1309(C—O),1236(O—H)
1.41(s,18H),1.61(br s,2H),2.00(br s,2H),2.82(t,2H),3.05(br s,2H),3.44(m,4H),3.94(br s,2H),4.35(br s,2H),4.92(s,1H),5.22(s,1H),7.19(s,2H),7.42(m,6H),7.83(br s,4H),13.37(s,1H),13.95(s,1H),7.25(s,HCCl3)
W08
3624(O—H),2956(CH3∶C—H),2436(N+—H),1310(C—O),1234(O—H)
, 百拇医药
1.42(s,18H),1.63(br s,2H),2.00(br s,2H),2.83(t,2H),3.06(br s,2H),3.45(m,4H),3.96(br s,2H),4.32(br s,2H),4.94(s,1H),5.23(s,1H),7.20(s,2H),7.44(m,5H),7.84(br s,4H),13.36(s,1H),13.97(s,1H),7.26(s,HCCl3)
W09
3617(O—H),2958(CH3∶C—H),2428(N+—H),1606,1513(Ar∶ CC ),1308(C—O),1235(O—H)
1.42(s,18H),1.70(m,2H),2.00(s,2H),2.84(t,2H),3.12(br s,2H),3.49(br s,4H),3.95(br s,2H),4.24(br s,2H),5.20(s,1H),5.23(s,1H),7.12(m,2H),7.19(s,2H),7.42(m,3H),7.99(br s,4H),13.23(s,1H),14.24(s,1H)
, http://www.100md.com
W10
3634(O—H),2958(CH3∶C—H),2396(N+—H),1316(C—O),1234(O—H)
1.43(s,36H),3.26(br s,8H),3.60(m,4H),4.00(br s,4H),5.37(s,2H),7.25(s,4H),13.72(s,1H),7.28(s,HCCl3)
W11
3633(O—H),2958(CH3∶C—H),2362(N+—H),1312(C—O),1233(O—H)
1.42(s,36H),2.14(m,4H),2.93(t,4H),3.42(t,4H),3.52(m,4H),4.02(m,4H),5.27(s,2H),7.21(s,4H),3.58(s,1H),7.26(s,HCCl3)
, 百拇医药
W12
3635(O—H),2955(CH3∶C—H),2364(N+—H),1314(C—O),1233(O—H)
1.37(s,36H),1.58(m,4H),1.77(br s,4H),2.87(t,4H),3.11(br s,4H),3.30(br s,4H),3.58(m,4H),7.08(s,4H),11.30(s,1H),2.50(H—DMSO-d5)
W13
3639(O—H),2954(CH3∶C—H),2331(N+—H),1312(C—O),1232(O—H)
1.36(s,36H),1.49~1.68(m,16H),2.83(t,4H),3.08(br s,4H),3.45(br s,4H),3.68(br s,4H),7.07(d,4H),11.62(s,1H),2.50(t,H—DMSO-d5)
, 百拇医药
2 合成实验
熔点用毛细管法测定,温度未经校正.红外光谱采用Bruker IFS 55 型仪器测定,溴化钾压片.核磁共振氢谱采用Bruker AC(E)-250 NMR仪和Bruker ARX-300 NMR仪测定,TMS为内标.元素分析采用Carlo Erba-1106型元素分析仪测定.
2.1 1-〔2-(3,5-二叔丁基-4-羟基苯基)硫〕乙基-4-二苯甲基哌嗪盐酸盐(W01)的制备
参考文献〔5〕制备2,6-二叔丁基-4-巯基苯酚,收率:90.5%,mp 86~88℃.
将2.4 g(0.01 mol)2,6-二叔丁基-4-巯基苯酚加入10 mL1,2-二溴乙烷中,加入2 mL三乙胺及0.1 g碘化钾,室温搅拌8 h,水洗一次,稀盐酸洗,再水洗两次,干燥,蒸出过量1,2-二溴乙烷,残留物备用.
, http://www.100md.com
取2.0 g(0.008 mol)二苯甲基哌嗪加入上述残留物中,加入2 mL三乙胺、0.1 g碘化钾及15 mL甲苯,回流14 h,用盐酸处理后,乙醇-水重结晶,得2.0 g白色晶体,收率:34.0%,mp 115~116℃.
W02~W13参考W01的制备方法制备,其收率及熔点见表1.
3 药理实验
3.1 抗变态反应药理实验结果与讨论
选定DNCB诱导的小鼠接触性皮炎药理模型〔3〕,对8个化合物进行了抗变态活性测定,结果见表3.
Tab.3 Effects of W-compounds on the delayed-type hypersensitivity responses to DNCB in mice
, 百拇医药
Treatment
Dose/mg.kg-1
Animal number
Ear swelling
(x±s)
Inhibition/%
P
control
-
10
33.2±6.1
, http://www.100md.com -
-
hydrocortisone
20×5
10
13.4±8.6
59.7
<0.01
W01
100×5
8
16.4±7.2
48.8
, 百拇医药
<0.01
W02
100×5
8
26.0±6.3
6.9
>0.05
W03
100×5
8
15.4±9.5
53.4
<0.01
, http://www.100md.com
W04
100×5
8
28.5±9.0
10.6
>0.05
W05
100×5
8
29.6±7.7
7.1
>0.05
W06
, http://www.100md.com
100×5
8
27.9±8.3
15.9
>0.05
W07
100×5
7
25.4±9.2
23.3
<0.05
W09
100×5
, 百拇医药
7
14.2±4.9
57.1
<0.01
与空白组对照,其中W01,W03,W07及W09的抑制率具有显著性.W01,W03和W09的抑制率较高,P<0.01,与氢化可的松活性相当.分析其结构,说明在该类化合物中,碳链为两个碳和四个碳时活性较高,并且含有氟原子的化合物活性较强.
3.2 抗炎药理实验结果及讨论
抗炎药理实验选用巴豆油致小鼠耳廓肿胀法〔4〕,结果见表4.Tab.4 Influences on crotonoil-induced ear swelling
Treatment
, 百拇医药
Dose/mg.kg-1
Animal number
Footpad swelling
(x±s)
Inhibition/%
P
control
-
8
24.19±3.44
-
W03
, 百拇医药
150
8
24.06±3.99
0.54
>0.05
W10
150
8
27.55±5.43
0
>0.05
indometacin
20
, 百拇医药
8
16.56±2.82
31.5
<0.01
ibuprofen
100
8
22.69±4.75
6.2
>0.05
抗炎药理实验结果表明W03和W10没有抗炎活性.
作者简介:王立升 通讯联系人
, 百拇医药
参考文献
1 王泽民,康葵,冯梅.当代结构药物全集.北京:北京科学技术出版社,1993.1977~2040
2 Mueller RA.Novel heterocyclic amides.EP 190685.1986-08-13
3 戴岳,杭秉茜,孟庆玉,等.齐墩果酸对变态反应的抑制作用.中国药理学报,1988,9(6):562~565
4 徐叔云,卞如濂,陈修.药理实验方法学.第二版.北京:人民卫生出版社,1994.719
5 Fuzisawa T,Hata K,Kojima T.The sulfurization of sterically hindered phenol with sulfur.A convenient synthesis of 4,4′-thio-bis-〔2,6-dialkylphenols〕and 2,6-dialkyl-4-mercaptophenols.Synthesis,1973,1:38~39
收稿日期:1999-08-09, http://www.100md.com