4-S-(5″-烃基-4″-氨基-1″,2″,4″-三唑-3″-基)-4-去氧-4′-去甲基表鬼臼毒素衍生物的合成及抗肿瘤活性
作者:鲁宽科 陈耀祖1
单位:(北京医科大学应用药物研究所,北京 100083; 1兰州大学应用有机国家重点实验室,兰州 730000)
关键词:鬼臼毒素;1,2,4三唑;抗肿瘤活性
药学学报990115.htm 许多有显著抗肿瘤活性的鬼臼毒素类化合物,其母核C-4侧链上往往连接有刚性较强的脂环或芳香环结构,而且侧链多含有一定数量的杂原子[1~3]。另外,三氮唑类化合物大都有广泛的生物活性,如抗菌[4~5]、抗病毒[6]、抗肿瘤[7]等,据此,我们设计并合成了8个三氮唑杂环取代的表鬼臼毒素衍生物,以期寻找活性高、毒副作用小的鬼臼毒素类药物,并进一步考察此类化合物的构效关系。
合成路线如图1所示,三氮唑3a~3h和4′-去甲基-表鬼臼毒2分别按文献[8,9]方法合成;我们选择三氟乙酸作为缩合剂,基于它不仅能催化缩合反应,而且能保护三唑上的氨基官能团,使其不能充当进攻基团;最后一步缩合反应显然经历了一个SN1历程,4′-去甲基-表鬼臼毒的C-4位上很容易形成一个苄基型碳正离子,由于C-1位有庞大的芳环,加之,进攻的基团也较大,可以预料,这个反应有很强的立体选择性,使C-4β-构型成为主要产物,事实确实如此,在TLC上几乎看不到C-4α-构型的产物。
, http://www.100md.com
SYNTHESIS AND ANTITUMOR ACTIVITIES OF 4β-S-(5″-ALKYL-
4
4″-AMINO-1″,2″,4″-TRIAZOLE-3″-YL)-4-DEOXY-
4′-O-DEMETHYL-EPIPODOPHYLLOTOXIN DERIVATIVES
Lu Kuanke (Lu KK) and Chen Yaozu (Chen YZ)1
(Institute of Applied Pharmaceutical Research, School of Pharmaceutical Science,Beijing Medical University, 100083;
, http://www.100md.com
1The State Key Laboratory of Applied Organic Chemistry, Lanzhou University, 730000)
ABSTRACT AIM: In order to search for podophyllotoxin analogue agents with fewer side effects and improved activity, the podophyllotoxin derivatives are to be synthesized. METHODS: Eight 4′-demethyl-podophyllotoxin analogues with 4β-S-triazoles have been synthesized from 4′-demethyl-podophyllotoxin. RESULTS: Eight 4′-demethyl-podophyllotoxin derivatives possessing 4β-S-triazoles have been synthesized and their antitumor activities were screened in vitro against HL-60, BGC-823, BcaP, KB and K562 cells. CONCLUSION: The results indicated that these compounds showed high biological activity only towards K562 cells.
, http://www.100md.com
KEY WORDS podophyllotoxin analogues; triazoles; antitumor activity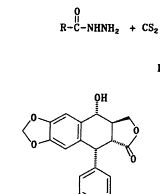
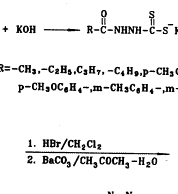

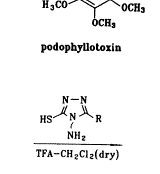
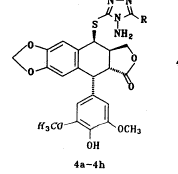
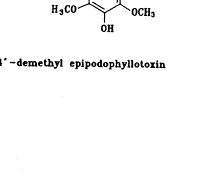
Scheme 1 Route of synthesis of compounds 4a~4h.
对表1所列的化合物进行了体外人白血病HL-60、人红白血病K562、人胃癌BGC-823、人乳腺癌Bcap、人鼻咽癌KB等肿瘤细胞株活性实验,结果(表2)表明,这些化合物对人红白血病K562的活性较强,而对其他肿瘤细胞的活性一般或较弱,对人红白血病K562肿瘤细胞来说,三氮唑环上的取代基对标题化合物的抗肿瘤活性影响较大,含烷基的化合物的活性高于含芳香基的化合物。对人白血病HL-60肿瘤细胞来说,正好相反;对其他肿瘤细胞来说,三氮唑环上的取代基对抗肿瘤活性影响较小。
, 百拇医药
Tab 1 Physical data of compounds 4a~4h Compd
R
Formula
MP/℃
J3,4
Elemental analysis / %
C
H
N
Calc
Found
, 百拇医药 Calc
Found
Calc
Found
4a
-CH3
C24H24N4O7S.1.5H2O
161~162
3.6
47.78
47.72
, 百拇医药
4.31
4.01
8.57
8.62
4b
-C2H5
C25H26N4O7S
167~169
3.4
50.62
50.92
, 百拇医药
4.25
4.22
8.75
8.60
4c
-C3H7
C26H28N4O7S
168~169
3.3
51.37
51.16
, http://www.100md.com
4.46
4.31
8.56
8.65
4d
-C4H9
C27H30N4O7S
165~168
3.3
52.09
52.31
, 百拇医药
4.67
4.77
8.37
8.30
4e
p-CH3C6H4-
C30H28N4O7S.H2O
200~204
4.0
59.68
, http://www.100md.com
59.85
4.66
4.48
9.60
9.44
4f
p-CH3OC6H4-
C29H34N4O7S.H2O
194~197
-
, 百拇医药
58.09
58.41
4.54
4.38
9.34
9.14
4g
m-CH3C6H4-
C29H34N4O7S.H2O
118~120
, http://www.100md.com
4.2
59.40
59.50
4.98
5.17
9.23
9.20
4h
m-FC6H4-
C29H34N4O7S
188~190
, 百拇医药
4.1
58.78
58.66
4.25
4.49
9.45
9.08
Tab 2 Inhibition of in vitro tumor cell growth by epipodophyllotoxin derivatives (4a~4h) Compd
Concentration/mol.L-1
Inhibition rate / %*
, 百拇医药
HL-60
K562
BGC-823
BcaP
KB
4a
10-6
37.9
68.76
-1.05
40.6
29.14
, 百拇医药
10-5
48.2
69.24
11.58
42.8
53.64
10-4
58.6
80.24
16.84
49.6
64.90
, http://www.100md.com
4b
10-6
48.2
76.15
1.05
37.5
23.18
10-5
55.1
83.10
30.53
43.6
, 百拇医药 52.98
10-4
58.6
86.83
40.00
54.1
74.83
4c
10-6
24.1
68.32
-10.53
, 百拇医药 38.3
-17.88
10-5
55.1
73.95
2.11
40.6
28.48
10-4
55.1
86.32
20.00
, http://www.100md.com 51.8
29.14
4d
10-6
22.3
60.71
48.31
-
47.02
10-5
49.6
72.86
50.56
, http://www.100md.com
-
67.55
10-4
58.6
79.07
56.18
-
74.83
4e
10-6
63.04
35.5
40.45
, 百拇医药
31.9
25.12
10-5
64.39
47.83
56.18
36.2
24.64
10-4
67.39
68.06
70.79
, 百拇医药
40.1
22.22
4f
10-6
47.82
6.07
50.56
25.1
8.21
10-5
47.82
45.23
, 百拇医药
51.69
28.5
19.32
10-4
76.08
64.88
82.02
36.0
25.60
4g
10-6
43.47
, 百拇医药
14.09
64.04
23.1
31.88
10-5
63.04
26.30
66.29
35.3
35.75
10-4
67.39
, 百拇医药
57.23
69.27
39.4
49.76
4h
10-6
41.3
51.01
43.82
26.6
17.39
10-5
, 百拇医药
50.0
53.61
61.80
28.6
20.29
10-4
56.5
76.16
65.17
35.9
38.16
a) Results obtained after 72 h; b) - No activity.实验部分
, 百拇医药
熔点在Kofler显微熔点仪上测定(温度未校正);IR在NICOLET-5DX型红外光谱仪上测定(KBr压片);1HNMR在Bruker-80A型核磁共振仪上测定,TMS内标;元素分析在Carlo-Erba元素自动分析仪上测定;旋光在J-20C型分光偏振仪上测定。
4a~4h的合成通法
4′-去甲基表鬼臼毒2(200 mg,0.5 mmol)溶于干燥的二氯甲烷(8 ml)中,冰盐浴至-10℃,在搅拌下加入三氮唑3(0.5 mmol),然后,滴入几滴CF3COOH,搅拌1~3 h,反应完全后(TLC检测),减压蒸去溶剂,残留物经乙醇重结晶或硅胶柱分离得产物4。它们的波谱数据如下:
4a 收率:89%,mp 161℃~162℃,[α]D20 -5.04°;IR(KBr):3360~3008(b,-NH2,-OH),1784.1,1671.1,1516.3,1483.1,1229.2,1191.8,1115.5,1034.5,935.33 cm-1;1HNMR(acetone-d6):7.02(s,1H,5-H),6.51(s,1H,8-H),6.36(s,2H,2′-H,6′-H),6.00(s,2H,OCH2O),5.43(d,1H,J=3.6 Hz,4-H),4.56(d,1H,J=4.0 Hz,1-H),3.97~4.4(m,2H,11-H),3.71(s,6H,3′-OCH3,5′-OCH3),3.0~3.3(m,2H,2-H,3-H),2.6(t,2H,-NH2),1.9(s,3H,-CH3)。
, http://www.100md.com
4b 收率:92%,mp 167℃~169℃,[α]D20 +46.2°;IR(KBr):3346.1,3310.9(双峰,-NH2),3212.5~3135(b,OH),1784.1,1673.41,1610.7,1516.7,1483.3,1232.7,1192.5,1114.1,1036.2,937.68 cm-1;1HNMR(acetone-d6):7.04(s,1H,5-H),6.51(s,1H,8-H),6.38(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.46(d,1H,J=3.4 Hz,4-H),4.60(d,1H,J=4.5 Hz,1-H),4.0~4.4(m,2H,11-H),3.71(s,6H,3′-OCH3,5′-OCH3),3.1~3.5(m,2H,2-H,3-H),2.6(t,2H,-NH2),1.76(q,2H,-CH2),0.96(t,3H,-CH3)。
, 百拇医药
4c 收率:89%,mp 168℃~169℃,[α]D20 +1.97°;IR(KBr):3346.1,3310.9(双峰,-NH2),3212.5(b,-OH),1785.1,1671.7,1611.9,1517.3,1483.4,1225.8,1192.0,1035.7,937.23 cm-1;1HNMR(acetone-d6):7.04(s,1H,5-H),6.51(s,1H,8-H),6.36(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.40(d,1H,J=3.3 Hz,4-H),4.60(d,1H,J=4.4 Hz,1-H),3.95~4.30(m,2H,11-H),3.70(s,6H,3′-OCH3,5′-OCH3),3.2~3.6(m,4H,2-H,3-H,CH2),2.5~2.9(m,4H,-NH2,CH2),1.76(q,2H,-CH2),1.27(t,3H,-CH3)。
, 百拇医药
4d 收率:76%,mp 165℃~168℃,[α]D20 -3.46°;IR(KBr):3367.2,3360~3212.5(双峰,-NH2),1780.9,1670.6,1482.6,1226.7,1191.2,1115.2,1035.0,936.36 cm-1;1HNMR(acetone-d6):7.04(s,1H,5-H),6.51(s,1H,8-H),6.38(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.41(d,1H,J=3.4 Hz,4-H),4.59(d,1H,J=4.4 Hz,1-H),4.10~4.40(m,2H,11-H),3.71(s,6H,3′-OCH3,5′-OCH3),3.3~3.4(m,2H,2-H,3-H),2.62(t,2H,-NH2),1.2~1.9(m,6H,-CH2CH2CH2-),0.95(t,3H,-CH3)。
, 百拇医药
4e 收率:85%,mp 200℃~204℃,[α]D20 -202.6°;IR(KBr):3430.5,3318,3170.3~3149.2(双峰,-NH2),1759.1,1614.0,1456.0,1218.7,1118.9,1032.7,921.22 cm-1;1HNMR(CDCl3):7.86(d,2H,J=7.8 Hz,2″-H,6″-H),7.33(d,2H,J=7.8 Hz,3″-H,5″-H),6.96(s,1H,5-H),6.51(s,1H,8-H),6.33(s,2H,2′-H,6′-H),5.98(s,2H,OCH2O),5.59(d,1H,J=4.0 Hz,4-H),4.4~4.8(m,4H,1-H,11-H,4′-OH),4.01(m,1H,2-H),3.81(s,6H,3′-OCH3,5′-OCH3),3.63(m,1H,3-H),3.26(s,2H,-NH2),2.44(s,3H,CH3)。
, http://www.100md.com
4f 收率:88%,mp 194℃~197℃,[α]D20 +364.8°;IR(KBr):3430.5~3156.2(b,-NH2,-OH),1761.7,1613.2,1515.4,1481.0,1243.9,1118.5,1032.2,921.79 cm-1;1HNMR(CDCl3):7.97(d,2H,J=8.5 Hz,2″-H,6″-H),7.02(d,2H,J=8.5 Hz,3″-H,5″-H),7.01(s,1H,5-H),6.51(s,1H,8-H),6.33(s,2H,2′-H,6′-H),5.98(s,2H,OCH2O),5.56(s,1H,4′-OH),4.4~4.9(m,4H,4-H,1-H,11-H),4.05(m,1H,2-H),3.88(s,3H-OCH3),3.81(s,6H,3′-OCH3,5′-OCH3),3.56(m,1H,3-H),3.29(s,2H,-NH2)。
, 百拇医药
4g 收率:85%,mp 118℃~120℃,[α]D20 -214.0°;IR(KBr):3353.1(b,-NH2,-OH),1763.8,1611.6,1517.4,1481.5,1223.9,1112.6,1035.9,932.43 cm-1;1HNMR(CDCl3):7.6~7.2(m,4H,苯环氢),7.05(s,1H,5-H),6.50(s,1H,8-H),6.26(s,2H,2′-H,6′-H),6.02(s,2H,OCH2O),5.42(d,1H,J=4.21 Hz,4-H),4.6~4.2(m,3H,1-H,11-H),3.63(s,6H,3′-OCH3,5′-OCH3),3.36(s,3H,CH3),5.03(s,1H,4′-OH),3.25(s,2H,-NH2),3.15~3.05(m,2H,2-H,3-H)。
, 百拇医药
4h 收率:82%,mp 188℃~190℃,[α]D20 -108.2°;IR(KBr):3353.1(b,-NH2,-OH),1764.1,1611.4,151.8,1480.9,1224.6,1112.0,1036.2,932.15 cm-1;1HNMR(acetone-d6):8.0~7.4(m,4H,苯环氢),7.12(s,1H,5-H),6.53(s,1H,8-H),6.41(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.55(s,1H,4′-OH),4.64(d,1H,J=4.1 Hz,4-H),4.32(d,1H,J=6.7 Hz,1-H),3.68(s,6H,3′-OCH3,5′-OCH3),3.66~3.20(m,4H,11-H,2-H,3-H),3.05(2H,-NH2)。
, 百拇医药
致谢 药理实验由北京医科大学天然与仿生药物国家重点实验室代做。
Tel:(01)62014945,Fax:(010)62091720,E-mail:gzw@mail.bjm.edu.cn
参考文献
1 Wang ZQ, Kuo YH, Schnur D, et al. Antitumor agents 113. New 4β arylmino derivatives of 4′-O-demethylepipodophyllotoxin and related compounds as potent inhibitors of human DNA topoisomerase II. J Med Chem, 1990,33∶2660
2 尹述凡,王志兴,王天都,等.鬼臼毒素类化合物分子作用模式与立体化学研究的现状.中国医药工业杂志,1991,22∶234
, http://www.100md.com
3 尹述凡,庄武,马维勇,等.4-O-卤代酰基-4′-去甲基表鬼臼毒素类似物的合成与抗肿瘤活性.药学学报,1993,28∶758
4 Senguta AK, Gupta AA. Synthesis of some indole derivatives as potential antibacterial agents Indian. J Chem, 1983,22B∶263
5 Nuesslein L, Baument D, et al. France Demande, 2463133, CA 1981,95,220075e
6 Malinoski F, Stollar V. Inhibitors of IMP dehydrogenase prevent sindbisirus replication and reduce FTP levels in aedes albopictus cells. Virology, 1981,110∶281
, 百拇医药
7 Miyahara M, Nakadate M. Antitumor activity of 2-acylamino-1,3,4-thiadiazoles and related compounds. Chem Pharm Bull, 1982,30∶4402
8 张自义,赵岚,李明.3-烷基-6芳亚甲基均三唑并[3,4,b]-1,3,4-噻二唑的合成.有机化学,1994,14∶80
9 Kuhn M, Kekker CJ, Wartburg AV. Partalsynthesis von 4′-demethylepipodophyllotoxin. Helv Chim Acta, 1969,52∶948
10 Denizot F, Lang R. Rapid colorimetric assay for cell frowth and survival, Modifications to the tetrazolium dye procedure giving improved sensitivity. J Immunol Methods, 1986,89∶277
收稿日期: 1998-11-10, http://www.100md.com(鲁宽科 陈耀祖1)
单位:(北京医科大学应用药物研究所,北京 100083; 1兰州大学应用有机国家重点实验室,兰州 730000)
关键词:鬼臼毒素;1,2,4三唑;抗肿瘤活性
药学学报990115.htm 许多有显著抗肿瘤活性的鬼臼毒素类化合物,其母核C-4侧链上往往连接有刚性较强的脂环或芳香环结构,而且侧链多含有一定数量的杂原子[1~3]。另外,三氮唑类化合物大都有广泛的生物活性,如抗菌[4~5]、抗病毒[6]、抗肿瘤[7]等,据此,我们设计并合成了8个三氮唑杂环取代的表鬼臼毒素衍生物,以期寻找活性高、毒副作用小的鬼臼毒素类药物,并进一步考察此类化合物的构效关系。
合成路线如图1所示,三氮唑3a~3h和4′-去甲基-表鬼臼毒2分别按文献[8,9]方法合成;我们选择三氟乙酸作为缩合剂,基于它不仅能催化缩合反应,而且能保护三唑上的氨基官能团,使其不能充当进攻基团;最后一步缩合反应显然经历了一个SN1历程,4′-去甲基-表鬼臼毒的C-4位上很容易形成一个苄基型碳正离子,由于C-1位有庞大的芳环,加之,进攻的基团也较大,可以预料,这个反应有很强的立体选择性,使C-4β-构型成为主要产物,事实确实如此,在TLC上几乎看不到C-4α-构型的产物。
, http://www.100md.com
SYNTHESIS AND ANTITUMOR ACTIVITIES OF 4β-S-(5″-ALKYL-
4
4″-AMINO-1″,2″,4″-TRIAZOLE-3″-YL)-4-DEOXY-
4′-O-DEMETHYL-EPIPODOPHYLLOTOXIN DERIVATIVES
Lu Kuanke (Lu KK) and Chen Yaozu (Chen YZ)1
(Institute of Applied Pharmaceutical Research, School of Pharmaceutical Science,Beijing Medical University, 100083;
, http://www.100md.com
1The State Key Laboratory of Applied Organic Chemistry, Lanzhou University, 730000)
ABSTRACT AIM: In order to search for podophyllotoxin analogue agents with fewer side effects and improved activity, the podophyllotoxin derivatives are to be synthesized. METHODS: Eight 4′-demethyl-podophyllotoxin analogues with 4β-S-triazoles have been synthesized from 4′-demethyl-podophyllotoxin. RESULTS: Eight 4′-demethyl-podophyllotoxin derivatives possessing 4β-S-triazoles have been synthesized and their antitumor activities were screened in vitro against HL-60, BGC-823, BcaP, KB and K562 cells. CONCLUSION: The results indicated that these compounds showed high biological activity only towards K562 cells.
, http://www.100md.com
KEY WORDS podophyllotoxin analogues; triazoles; antitumor activity

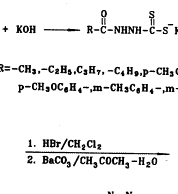




Scheme 1 Route of synthesis of compounds 4a~4h.
对表1所列的化合物进行了体外人白血病HL-60、人红白血病K562、人胃癌BGC-823、人乳腺癌Bcap、人鼻咽癌KB等肿瘤细胞株活性实验,结果(表2)表明,这些化合物对人红白血病K562的活性较强,而对其他肿瘤细胞的活性一般或较弱,对人红白血病K562肿瘤细胞来说,三氮唑环上的取代基对标题化合物的抗肿瘤活性影响较大,含烷基的化合物的活性高于含芳香基的化合物。对人白血病HL-60肿瘤细胞来说,正好相反;对其他肿瘤细胞来说,三氮唑环上的取代基对抗肿瘤活性影响较小。
, 百拇医药
Tab 1 Physical data of compounds 4a~4h Compd
R
Formula
MP/℃
J3,4
Elemental analysis / %
C
H
N
Calc
Found
, 百拇医药 Calc
Found
Calc
Found
4a
-CH3
C24H24N4O7S.1.5H2O
161~162
3.6
47.78
47.72
, 百拇医药
4.31
4.01
8.57
8.62
4b
-C2H5
C25H26N4O7S
167~169
3.4
50.62
50.92
, 百拇医药
4.25
4.22
8.75
8.60
4c
-C3H7
C26H28N4O7S
168~169
3.3
51.37
51.16
, http://www.100md.com
4.46
4.31
8.56
8.65
4d
-C4H9
C27H30N4O7S
165~168
3.3
52.09
52.31
, 百拇医药
4.67
4.77
8.37
8.30
4e
p-CH3C6H4-
C30H28N4O7S.H2O
200~204
4.0
59.68
, http://www.100md.com
59.85
4.66
4.48
9.60
9.44
4f
p-CH3OC6H4-
C29H34N4O7S.H2O
194~197
-
, 百拇医药
58.09
58.41
4.54
4.38
9.34
9.14
4g
m-CH3C6H4-
C29H34N4O7S.H2O
118~120
, http://www.100md.com
4.2
59.40
59.50
4.98
5.17
9.23
9.20
4h
m-FC6H4-
C29H34N4O7S
188~190
, 百拇医药
4.1
58.78
58.66
4.25
4.49
9.45
9.08
Tab 2 Inhibition of in vitro tumor cell growth by epipodophyllotoxin derivatives (4a~4h) Compd
Concentration/mol.L-1
Inhibition rate / %*
, 百拇医药
HL-60
K562
BGC-823
BcaP
KB
4a
10-6
37.9
68.76
-1.05
40.6
29.14
, 百拇医药
10-5
48.2
69.24
11.58
42.8
53.64
10-4
58.6
80.24
16.84
49.6
64.90
, http://www.100md.com
4b
10-6
48.2
76.15
1.05
37.5
23.18
10-5
55.1
83.10
30.53
43.6
, 百拇医药 52.98
10-4
58.6
86.83
40.00
54.1
74.83
4c
10-6
24.1
68.32
-10.53
, 百拇医药 38.3
-17.88
10-5
55.1
73.95
2.11
40.6
28.48
10-4
55.1
86.32
20.00
, http://www.100md.com 51.8
29.14
4d
10-6
22.3
60.71
48.31
-
47.02
10-5
49.6
72.86
50.56
, http://www.100md.com
-
67.55
10-4
58.6
79.07
56.18
-
74.83
4e
10-6
63.04
35.5
40.45
, 百拇医药
31.9
25.12
10-5
64.39
47.83
56.18
36.2
24.64
10-4
67.39
68.06
70.79
, 百拇医药
40.1
22.22
4f
10-6
47.82
6.07
50.56
25.1
8.21
10-5
47.82
45.23
, 百拇医药
51.69
28.5
19.32
10-4
76.08
64.88
82.02
36.0
25.60
4g
10-6
43.47
, 百拇医药
14.09
64.04
23.1
31.88
10-5
63.04
26.30
66.29
35.3
35.75
10-4
67.39
, 百拇医药
57.23
69.27
39.4
49.76
4h
10-6
41.3
51.01
43.82
26.6
17.39
10-5
, 百拇医药
50.0
53.61
61.80
28.6
20.29
10-4
56.5
76.16
65.17
35.9
38.16
a) Results obtained after 72 h; b) - No activity.实验部分
, 百拇医药
熔点在Kofler显微熔点仪上测定(温度未校正);IR在NICOLET-5DX型红外光谱仪上测定(KBr压片);1HNMR在Bruker-80A型核磁共振仪上测定,TMS内标;元素分析在Carlo-Erba元素自动分析仪上测定;旋光在J-20C型分光偏振仪上测定。
4a~4h的合成通法
4′-去甲基表鬼臼毒2(200 mg,0.5 mmol)溶于干燥的二氯甲烷(8 ml)中,冰盐浴至-10℃,在搅拌下加入三氮唑3(0.5 mmol),然后,滴入几滴CF3COOH,搅拌1~3 h,反应完全后(TLC检测),减压蒸去溶剂,残留物经乙醇重结晶或硅胶柱分离得产物4。它们的波谱数据如下:
4a 收率:89%,mp 161℃~162℃,[α]D20 -5.04°;IR(KBr):3360~3008(b,-NH2,-OH),1784.1,1671.1,1516.3,1483.1,1229.2,1191.8,1115.5,1034.5,935.33 cm-1;1HNMR(acetone-d6):7.02(s,1H,5-H),6.51(s,1H,8-H),6.36(s,2H,2′-H,6′-H),6.00(s,2H,OCH2O),5.43(d,1H,J=3.6 Hz,4-H),4.56(d,1H,J=4.0 Hz,1-H),3.97~4.4(m,2H,11-H),3.71(s,6H,3′-OCH3,5′-OCH3),3.0~3.3(m,2H,2-H,3-H),2.6(t,2H,-NH2),1.9(s,3H,-CH3)。
, http://www.100md.com
4b 收率:92%,mp 167℃~169℃,[α]D20 +46.2°;IR(KBr):3346.1,3310.9(双峰,-NH2),3212.5~3135(b,OH),1784.1,1673.41,1610.7,1516.7,1483.3,1232.7,1192.5,1114.1,1036.2,937.68 cm-1;1HNMR(acetone-d6):7.04(s,1H,5-H),6.51(s,1H,8-H),6.38(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.46(d,1H,J=3.4 Hz,4-H),4.60(d,1H,J=4.5 Hz,1-H),4.0~4.4(m,2H,11-H),3.71(s,6H,3′-OCH3,5′-OCH3),3.1~3.5(m,2H,2-H,3-H),2.6(t,2H,-NH2),1.76(q,2H,-CH2),0.96(t,3H,-CH3)。
, 百拇医药
4c 收率:89%,mp 168℃~169℃,[α]D20 +1.97°;IR(KBr):3346.1,3310.9(双峰,-NH2),3212.5(b,-OH),1785.1,1671.7,1611.9,1517.3,1483.4,1225.8,1192.0,1035.7,937.23 cm-1;1HNMR(acetone-d6):7.04(s,1H,5-H),6.51(s,1H,8-H),6.36(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.40(d,1H,J=3.3 Hz,4-H),4.60(d,1H,J=4.4 Hz,1-H),3.95~4.30(m,2H,11-H),3.70(s,6H,3′-OCH3,5′-OCH3),3.2~3.6(m,4H,2-H,3-H,CH2),2.5~2.9(m,4H,-NH2,CH2),1.76(q,2H,-CH2),1.27(t,3H,-CH3)。
, 百拇医药
4d 收率:76%,mp 165℃~168℃,[α]D20 -3.46°;IR(KBr):3367.2,3360~3212.5(双峰,-NH2),1780.9,1670.6,1482.6,1226.7,1191.2,1115.2,1035.0,936.36 cm-1;1HNMR(acetone-d6):7.04(s,1H,5-H),6.51(s,1H,8-H),6.38(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.41(d,1H,J=3.4 Hz,4-H),4.59(d,1H,J=4.4 Hz,1-H),4.10~4.40(m,2H,11-H),3.71(s,6H,3′-OCH3,5′-OCH3),3.3~3.4(m,2H,2-H,3-H),2.62(t,2H,-NH2),1.2~1.9(m,6H,-CH2CH2CH2-),0.95(t,3H,-CH3)。
, 百拇医药
4e 收率:85%,mp 200℃~204℃,[α]D20 -202.6°;IR(KBr):3430.5,3318,3170.3~3149.2(双峰,-NH2),1759.1,1614.0,1456.0,1218.7,1118.9,1032.7,921.22 cm-1;1HNMR(CDCl3):7.86(d,2H,J=7.8 Hz,2″-H,6″-H),7.33(d,2H,J=7.8 Hz,3″-H,5″-H),6.96(s,1H,5-H),6.51(s,1H,8-H),6.33(s,2H,2′-H,6′-H),5.98(s,2H,OCH2O),5.59(d,1H,J=4.0 Hz,4-H),4.4~4.8(m,4H,1-H,11-H,4′-OH),4.01(m,1H,2-H),3.81(s,6H,3′-OCH3,5′-OCH3),3.63(m,1H,3-H),3.26(s,2H,-NH2),2.44(s,3H,CH3)。
, http://www.100md.com
4f 收率:88%,mp 194℃~197℃,[α]D20 +364.8°;IR(KBr):3430.5~3156.2(b,-NH2,-OH),1761.7,1613.2,1515.4,1481.0,1243.9,1118.5,1032.2,921.79 cm-1;1HNMR(CDCl3):7.97(d,2H,J=8.5 Hz,2″-H,6″-H),7.02(d,2H,J=8.5 Hz,3″-H,5″-H),7.01(s,1H,5-H),6.51(s,1H,8-H),6.33(s,2H,2′-H,6′-H),5.98(s,2H,OCH2O),5.56(s,1H,4′-OH),4.4~4.9(m,4H,4-H,1-H,11-H),4.05(m,1H,2-H),3.88(s,3H-OCH3),3.81(s,6H,3′-OCH3,5′-OCH3),3.56(m,1H,3-H),3.29(s,2H,-NH2)。
, 百拇医药
4g 收率:85%,mp 118℃~120℃,[α]D20 -214.0°;IR(KBr):3353.1(b,-NH2,-OH),1763.8,1611.6,1517.4,1481.5,1223.9,1112.6,1035.9,932.43 cm-1;1HNMR(CDCl3):7.6~7.2(m,4H,苯环氢),7.05(s,1H,5-H),6.50(s,1H,8-H),6.26(s,2H,2′-H,6′-H),6.02(s,2H,OCH2O),5.42(d,1H,J=4.21 Hz,4-H),4.6~4.2(m,3H,1-H,11-H),3.63(s,6H,3′-OCH3,5′-OCH3),3.36(s,3H,CH3),5.03(s,1H,4′-OH),3.25(s,2H,-NH2),3.15~3.05(m,2H,2-H,3-H)。
, 百拇医药
4h 收率:82%,mp 188℃~190℃,[α]D20 -108.2°;IR(KBr):3353.1(b,-NH2,-OH),1764.1,1611.4,151.8,1480.9,1224.6,1112.0,1036.2,932.15 cm-1;1HNMR(acetone-d6):8.0~7.4(m,4H,苯环氢),7.12(s,1H,5-H),6.53(s,1H,8-H),6.41(s,2H,2′-H,6′-H),6.01(s,2H,OCH2O),5.55(s,1H,4′-OH),4.64(d,1H,J=4.1 Hz,4-H),4.32(d,1H,J=6.7 Hz,1-H),3.68(s,6H,3′-OCH3,5′-OCH3),3.66~3.20(m,4H,11-H,2-H,3-H),3.05(2H,-NH2)。
, 百拇医药
致谢 药理实验由北京医科大学天然与仿生药物国家重点实验室代做。
Tel:(01)62014945,Fax:(010)62091720,E-mail:gzw@mail.bjm.edu.cn
参考文献
1 Wang ZQ, Kuo YH, Schnur D, et al. Antitumor agents 113. New 4β arylmino derivatives of 4′-O-demethylepipodophyllotoxin and related compounds as potent inhibitors of human DNA topoisomerase II. J Med Chem, 1990,33∶2660
2 尹述凡,王志兴,王天都,等.鬼臼毒素类化合物分子作用模式与立体化学研究的现状.中国医药工业杂志,1991,22∶234
, http://www.100md.com
3 尹述凡,庄武,马维勇,等.4-O-卤代酰基-4′-去甲基表鬼臼毒素类似物的合成与抗肿瘤活性.药学学报,1993,28∶758
4 Senguta AK, Gupta AA. Synthesis of some indole derivatives as potential antibacterial agents Indian. J Chem, 1983,22B∶263
5 Nuesslein L, Baument D, et al. France Demande, 2463133, CA 1981,95,220075e
6 Malinoski F, Stollar V. Inhibitors of IMP dehydrogenase prevent sindbisirus replication and reduce FTP levels in aedes albopictus cells. Virology, 1981,110∶281
, 百拇医药
7 Miyahara M, Nakadate M. Antitumor activity of 2-acylamino-1,3,4-thiadiazoles and related compounds. Chem Pharm Bull, 1982,30∶4402
8 张自义,赵岚,李明.3-烷基-6芳亚甲基均三唑并[3,4,b]-1,3,4-噻二唑的合成.有机化学,1994,14∶80
9 Kuhn M, Kekker CJ, Wartburg AV. Partalsynthesis von 4′-demethylepipodophyllotoxin. Helv Chim Acta, 1969,52∶948
10 Denizot F, Lang R. Rapid colorimetric assay for cell frowth and survival, Modifications to the tetrazolium dye procedure giving improved sensitivity. J Immunol Methods, 1986,89∶277
收稿日期: 1998-11-10, http://www.100md.com(鲁宽科 陈耀祖1)