电刺激POAH对IL-1β作用下兔VSA温敏神经元放电的影响
作者:董军 陆大祥 付咏梅 颜亮 谭敦勇 李楚杰
单位:(暨南大学医学院病理生理教研室, 广东 广州 510632)
关键词:隔核;视前区;下丘脑区,前;体温调节;白细胞介素-1;电生理学
中国病理生理杂志000904 [摘 要]目的和方法:为了从细胞水平进一步探讨发热时体温正、负调节中枢的调节机制,本实验采用微电极细胞外记录技术,在26只新西兰兔脑腹中隔区(ventral septal area, VSA)记录了温敏神经元单位放电,观察电刺激下丘脑视前区(preoptic anterior hypothalamus, POAH)对致热原IL-1β作用下兔VSA温敏神经元放电的影响。结果:(1)侧脑室注射的白介素-1β(IL-1β)能使VSA热敏神经元放电频率增加,冷敏神经元放电减少,而侧脑室注射等量人工脑脊液(ACSF)对热敏神经元和冷敏神经元的放电均无明显影响。(2)电刺激POAH可反转致热原IL-1β对VSA热敏神经元和冷敏神经元的上述作用。结论:在致热原作用下的体温调节中,正调节中枢POAH和负调节中枢VSA具有密切的相互作用。
, 百拇医药
[中图分类号] R364.6 [文献标识码] A
[文章编号] 1000-4718(2000)09-0783-05
Electrical stimulation of POAH alters firing rates of
IL-1β-treated thermosensitive neurons
in the VSA in rabbits*
DONG Jun, LU Da-xiang, FU Yong-mei, YAN Liang, TAN Dun-yong, LI Chu-jie△
(Department of Pathophysiology, Medical College of Jinan University, Guangzhou 510632,China)
, 百拇医药
[Abstract] AIM and METHODS: To investigate the functional connection between the preoptic anterior hypothalamus (POAH) and the ventral septal area (VSA) in fever mechanism, the firing rates of thermosensitive neurons in the VSA of 26 New Zealand white rabbits were recorded using extracellular microelectrode technique. RESULTS: The firing rates in both types of thermosensitive neurons in the VSA had no significant changes after intracerebroventricular (icv) injection of artificial cerebrospinal fluid(ACSF). When interleukin-1β (IL-1β) was given (icv), the firing rate of the warm-sensitive neurons was increased significantly and that of the cold-sensitive neurons was decreased remarkably. The effects of IL-1β on the changes of firing rate in thermosensitive neurons of the VSA were reversed by electrical stimulation of the POAH. CONCLUSION: The roles of positive and negative thermoregulatory centers in the interaction between the POAH and VSA are closely linked during endogenous pyrogen induced fever.
, http://www.100md.com
[MeSH] Septal nuclei; Preoptic area; Anterior Hypothalamic area; Body temperature regulation; Interleukin-1;Electrophysiology
[CLC number] R364.6 [Document code] A
INTRODUCTION
In an earlier report, we showed that the VSA plays a negative-regulation central role in thermoregulation during endogenous pyrogen induced fever. The firing rate of warm-sensitive neurons decreased and that of cold-sensitive neurons increased remarkably in the region of POAH in rabbits after icv. injection of IL-1β. These responses are appropriate for the increase in heat production and the decrease in heat loss. The effects could be reversed by electrical stimulation of the VSA[1], by which the responses are appropriate for the inhibiting heat production and promoting heat loss. In order to further confirm the functional connection between positive and negative regulatory center in fever mechanism, we investigated the effects of electrical stimulation of POAH on firing rates of IL-1β-treated thermosensitive neurons in the VSA by using extracellular microelectrode technique.
, http://www.100md.com
MATERIALS AND METHODS
Surgical procedure The experiments were performed on 26 New Zealand white rabbits of both sexes weighing 1.5-3.0 kg. The animals were anesthetized with 25% urethane (1.0 g/kg,iv). Supplemental doses of anesthetics were given when required. A midline incision was made on the ventral surface of the neck. The animal breathed spontaneously through a trachea tube and was fixed on a stereotaxic frame (Model IIc Jiangwan). Tubular thermodes (Φ1.2 mm) were placed in A0、A5 beyond midline in skull below. Tissue temperature was monitored by a thermistor probe (Φ1.2 mm) placed in a contralateral position symmetrical to that of the recording electrode. Stimulating electrode was insected into the POAH (Stereotaxic coordinates: A2.5、R2.5、H1) , guide cannulae were stereotaxically implanted into the lateral ventricle of rabbits (stereotaxic coordinates: P4.5、R4.0、H6.5). The lateral ventricle infusion was controlled by a microinjector (Shanghai). the environmental temperature was maintained at (22±2)℃during experiment,colon temperature was maintained at 37-39℃.
, http://www.100md.com
Electrophysiological recording Single-Unit recording was made with a glass microelectrode (<1 μm tip, DC resistance 8-15 MΩ, filled with 2% pontamine sky blue and acetate sodium 0.5 mol/L at pH 7.3-7.4. The microelectrode was advanced at the speed of 1μm/s by a micromanipulator (SM-21, Narishige) into the VSA (stereotaxic coordinates: A3-5, R0-3, H0-4) until the activity of an extracellular single unit was recorded. Singals were amplified and filtered by a microelectrode amplifier(MEZ-8300, Nihon Kohden). The amplified electrical signals was monitored on an oscilloscope and recorded by a tape recorder (Victor Company of Japan). Spikes were counted by a computer controled Physiological Signal Processing System(NSA-II, NanJing) and displayed in array density histograms.
, 百拇医药
A monopolar stimulating electrode was constructed from a stainless steel in sect pin (size=00 with a tipe exposure of 500 μm), with the use of a stimulator set to generate monophasic square-wave pulses of 0.5 ms duration, each site was stimulated electrically at a frequency of 50 Hz(30 s, 400 μA).
Experimental protocols After unit-signal activity stabilized 10 minutes,the thermal-response pattern of a unit in H0-4 was determined by recording the units firing rate as brain temperature was slowly raried twice at a rate of about 0.1-2℃/min by super constant temperature pump from about 34℃ to 42℃, neurons were classfied as warm-sensitive if they had a positive thermal coefficient of at least 0.8 impulse/(s*℃) [imps/(s*℃)] or cold-sensitive if they had a thermal coefficient of -0.6 imps/(s*℃) or less. IL-1β(10 mg/L) (BangDin Company of Beijing, China) was injected (icv). After the icv injection of ACSF 10 minutes, effects of electrical stimulation of POAH on the firing rate of neurons in the VSA were recorded.
, 百拇医药
Histological assay At the end of experiment, the dye filled in glass micropippets was diffused out with a negative direct current (20 μA). While the animals were deeply anesthetized, the brain was quickly removed and fixed in 10% formalin. Forzen brain tissue was sectioned in the coronal plane (20-40 μm) after 5-7 d, Histological verification was carried out with reference to Sawyers coordinates[2]. Data from those electrode tips not in the desired area were excluded.
, http://www.100md.com
Statistics Data were expressed as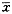 ±s and analyzed using paired Students t test. Differences were considered statistically significant as P<0.05.
±s and analyzed using paired Students t test. Differences were considered statistically significant as P<0.05.
RESULTS
Effects of IL-1β on the firing rate of warm-sensitive neurons in the VSA In 14 rabbits, eight spontaneously active single-units in desired area were recorded. After icv injection of IL-1β, the firing rate of warm-sensitive neurons were increased from (5.76±2.58) imps/s (before injection) to (5.80±2.10) imps/s(P>0.05),(8.92±3.73) imps/s(P<0.01), (13.58±6.37) imps/s(P<0.01) and (19.86±6.36) imps/s (P<0.01) at 1, 3,5 and 10 min after icv injection, respectively. However, the firing rate of warm-sensitive neurons had no significant changes (P<0.05) after icv injection of equivalent artificial cerebrospinal fluid (ACSF) (Fig 1). The results indicated that the firing rate of warm-sensitive neurons in the VSA increased remarkably when icv exposed to IL-1β.
, http://www.100md.com
Effects of electrical stimulation of POAH on the firing rate of IL-1β-treated warm-sensitive neurons in the VSA In 14 rabbits, eight spontaneously active single-units were recorded. After icv injection of IL-1β, the firing rate of warm-sensitive neurons increased remarkably. When the POAH was stimulated with current (400 μA), the firing rate of warm-sensitive neurons were decreased from (19.86±10.36) imps/s (before stimulation) to (7.67±5.74) imps/s (P<0.01), (4.19±2.18) imps/s (P<0.01), (1.37±0.36) imps/s (P<0.01), (3.34±2.43) imps/s (P<0.01) and (5.92±2.63) imps/s (P<0.01) at 1, 3, 5, 10 and 15 min after stimulation, respectively (Fig 1).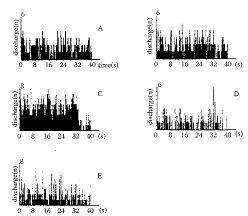
, 百拇医药
Fig 1 Effects of electrical stimulation POAH on discharge activity of pyrogen (IL-1β)-treated warm-sensitive neurons in VSA (spike array density).
A. spontaneous discharge; B. i.c.v. injection of ACSF;
C. i.c.v. injection of IL-1β; D. electrical stimulation POAH;
E. recover spontaneous discharge.
Effects of IL-1β on the firing rate of cold-sensitive neurons in the VSA In 12 rabbits, six spontaneously active single-units in desired area were recorded. After icv injection of IL-1β, the firing rate of cold-sensitive neurons were decreased from (2.45±0.38) imps/s (before injection) to (2.40±0.53) imps/s (P>0.05),(1.46±0.66) imps/s (P<0.05),(0.89±0.57) imps/s (P<0.01) and (0.49±0.40) imps/s (P<0.01) at 1, 3, 5 and 10 min after icv injection, respectively. However, The firing rate of cold-sensitive neurons had no significant changes (P<0.05) after icv injection of equivalent ACSF (Fig.2). The results indicated that the firing rate of cold-sensitive neurons in the VSA decreased remarkably when icv injection of IL-1β.
, http://www.100md.com
Effects of electrical stimulation of POAH on the firing rate of IL-1β-treated cold-sensitive neurons in the VSA In 12 rabbits, six spontaneously active single-unit in desired area were recorded. After icv injection of IL-1β, the firing rate of cold-sensitive neurons decreased remarkably. When the POAH was stimulated with current (400 μA), the firing rate of cold-sensitive neurons increased from (0.49±0.40) imps/s (before stimulation) to (5.53±4.42) imps/s (P<0.05), (8.9±3.80) imps/s (P<0.05),(18.88±0.21) imps/s (P<0.01)、(7.79±4.26) imps/s (P<0.01) and (2.91±0.62) imps/s (P<0.01) at 1, 3, 5, 10 and 15 min after POAH stimulation, respectively (Fig.2).
, 百拇医药
Fig 2 Effects of electrical stimulation POAH on discharge activity of pyrogen (IL-1β)-treated cold-sensitive neurons in the VSA (spike array density).
A. spontaneous discharge; B. i.c.v. injection of ACSF;
C. i.c.v. injection of IL-1β;
D. electrical stimulation of POAH;
E. recover spontaneous discharge.
, http://www.100md.com DISCUSSION
Considerable evidences have accumulated indicating that endogenous pyrogens (IL-1, TNF, IFN-γ、IL-6 etc) and central febrile mediators (PGE, cAMP, etc.) act principally on the central region of POAH (considered as positive thermoregulatory center) while endogenous antipyretics (AVP, α-MSH, etc.) act primary on the VSA and medial amygdaloid nucleus (considered as negative thermoregulatory center)[2-6]. The altitude of body temperature elevating during pyrogen induced fever results from the interaction of positive and negative regulation[7-9]. An early study with dissect construction had demonstrated that the locus of the POAH and VSA have thermosensitive neurons and widely fiber connections[10]. However, it remains to be elucidated whether this has some functional connection to fever mechanism. Our recent observation found that the firing rate of warm-sensitive neurons decreased and that of cold-sensitive neurons increased remarkably after icv injection of IL-1β. The effect of IL-1β on the thermosensitive neurons in the POAH was reversed by electrical stimulation of the VSA. These findings support the concept that positive and negative thermoregulatory centers are functionally connected. The results also indicate that the VSA plays a negative central role in response to the pyrogen. In the present investigation, we further confirmed that icv of IL-1β affected electrophysiological activity of thermosensitive neurons in the VSA, and electrical stimulation of POAH reversed the effect of IL-1β, indicating that the POAH plays a positive regulatory role. Collectively, these in vivo data reinforced the conclusion: (1) There are at least two types thermoregulatory positive and negative centers involved in fever mechanism. (2) The POAH and VSA are functionally connected.
, 百拇医药
There have been several attempts to study thermoregulation at level of single neuron. Recording firing rate reveals the presence of specialized cells that are very sensitive to imposed changes in local temperature. Our data showed that IL-1β diminished warm-sensitive neuron activity and enhanced cold-sensitive neuron in the POAH, suggesting that POAH is positive in response to the action of endogenous pyrogen and triggers the increase in heat production and decrease in heat lost. Furthermore, electrical stimulation of the VSA reversed the effect of IL-1β, revealing that the VSA has a negative functional connection to the thermosensitive neural activity in the POAH. The data offered in the present investigation further confirm this conclusion and strongly support the view point that the VSA is a negative thermoregulatory center for pyrogen-induced fever. Electrical stimulation of POAH reversed the effect of IL-1β on the neural activity in the VSA, gives a strong evidence that POAH has a positive functional connection to the VSA. Although it is still a question whether IL-1β has direct effect on thermosensitive neurons or connection by neural fibers between the POAH and the VSA remains to be elucidated, it is important that the functional relationship between these two regions has been electrical stitrophysiologically established for explaining fever mechanism.
, 百拇医药
[Foundation item] Work supported by the National Natural Science Foundation of China (No. 39600061); The Office of the Overseas Chinese Affairs of the State Council Scientific Research Foundation (No. 93-95-8)
△ To whom correspondence should be addressed
Tel: 020-85220253
[REFERENCES]
[1] Dong J,Li CJ, Lu DX, et al. Effects of electrical stimulation VSA on the firing of pyrogen-treated thermosensitive neurons in the region of POAH in rabbits[J]. Chin J Pathphysiol, 2000, 16(4): 301-303.
, 百拇医药
[2] Sawyer CH, Everett JW, Green JD. The rabbit diencephalons in stereotaxic coordinates[J]. J Comp Neurol, 1954, 101(2): 801-824.
[3] Federico P, Veale WL, Pittman QJ. Vasopressin-induced antipyresis in the medial amygdaloid nucleus of conscious rats[J]. Am J Physiol, 1992, 262(5Pt2): R901-908.
[4] Federico P, Malkinson TJ, Cooper KE, et al. Vasopressin perfusion within the medial amygdaloid nucleus attenuates prostaglandin fever in the urethane-anaesthetized rat[J]. Brain Res, 1992, 587(2):319-326.
, http://www.100md.com
[5] Yang YL, Wang XM, Shi SG,et al. The effect of microinjection of AVP and AVPMcAb into medial amygdaloid nucleus on endotoxin-induced fever in rabbits[J]. Chin J Appl Physiol, 1999, 15(1):62-65. (in Chinese with English abstract).
[6] Willian D, Ruwe. Electrical Stimulation of the paraventricular nucleus attenuates pyrogen fever in rabbit[J]. Brain Res, 1992, 588:181-190.
[7] Li CJ. Positive-negitive thermoregulation in fever[J]. Chin J Pathophysiol, 1994, 10(5):553-557.
, http://www.100md.com
[8] Zeisberger E, Roth J. Neurobiological concepts of fever generation and suppression[J]. Neuropsychobiology, 1993, 28(1-2):106-109.
[9] Cooper KE, Malkinson TJ, Monda M, et al. New neurobiogical concepts of the genesis of fever and of antipyresis[M]. In: Barfai I, Ottoson D eds. Neuroimmunology of Fever. New York: Pergaman Press, 1992. 225-234.
[10] Cheng ZX. Connection with nucleus and fiber in hypothalamus. Journal of Tianjing Medical College, 1983, 19(1):19-29.
[Received]2000-05-23 [Accepted] 2000-06-22, 百拇医药
单位:(暨南大学医学院病理生理教研室, 广东 广州 510632)
关键词:隔核;视前区;下丘脑区,前;体温调节;白细胞介素-1;电生理学
中国病理生理杂志000904 [摘 要]目的和方法:为了从细胞水平进一步探讨发热时体温正、负调节中枢的调节机制,本实验采用微电极细胞外记录技术,在26只新西兰兔脑腹中隔区(ventral septal area, VSA)记录了温敏神经元单位放电,观察电刺激下丘脑视前区(preoptic anterior hypothalamus, POAH)对致热原IL-1β作用下兔VSA温敏神经元放电的影响。结果:(1)侧脑室注射的白介素-1β(IL-1β)能使VSA热敏神经元放电频率增加,冷敏神经元放电减少,而侧脑室注射等量人工脑脊液(ACSF)对热敏神经元和冷敏神经元的放电均无明显影响。(2)电刺激POAH可反转致热原IL-1β对VSA热敏神经元和冷敏神经元的上述作用。结论:在致热原作用下的体温调节中,正调节中枢POAH和负调节中枢VSA具有密切的相互作用。
, 百拇医药
[中图分类号] R364.6 [文献标识码] A
[文章编号] 1000-4718(2000)09-0783-05
Electrical stimulation of POAH alters firing rates of
IL-1β-treated thermosensitive neurons
in the VSA in rabbits*
DONG Jun, LU Da-xiang, FU Yong-mei, YAN Liang, TAN Dun-yong, LI Chu-jie△
(Department of Pathophysiology, Medical College of Jinan University, Guangzhou 510632,China)
, 百拇医药
[Abstract] AIM and METHODS: To investigate the functional connection between the preoptic anterior hypothalamus (POAH) and the ventral septal area (VSA) in fever mechanism, the firing rates of thermosensitive neurons in the VSA of 26 New Zealand white rabbits were recorded using extracellular microelectrode technique. RESULTS: The firing rates in both types of thermosensitive neurons in the VSA had no significant changes after intracerebroventricular (icv) injection of artificial cerebrospinal fluid(ACSF). When interleukin-1β (IL-1β) was given (icv), the firing rate of the warm-sensitive neurons was increased significantly and that of the cold-sensitive neurons was decreased remarkably. The effects of IL-1β on the changes of firing rate in thermosensitive neurons of the VSA were reversed by electrical stimulation of the POAH. CONCLUSION: The roles of positive and negative thermoregulatory centers in the interaction between the POAH and VSA are closely linked during endogenous pyrogen induced fever.
, http://www.100md.com
[MeSH] Septal nuclei; Preoptic area; Anterior Hypothalamic area; Body temperature regulation; Interleukin-1;Electrophysiology
[CLC number] R364.6 [Document code] A
INTRODUCTION
In an earlier report, we showed that the VSA plays a negative-regulation central role in thermoregulation during endogenous pyrogen induced fever. The firing rate of warm-sensitive neurons decreased and that of cold-sensitive neurons increased remarkably in the region of POAH in rabbits after icv. injection of IL-1β. These responses are appropriate for the increase in heat production and the decrease in heat loss. The effects could be reversed by electrical stimulation of the VSA[1], by which the responses are appropriate for the inhibiting heat production and promoting heat loss. In order to further confirm the functional connection between positive and negative regulatory center in fever mechanism, we investigated the effects of electrical stimulation of POAH on firing rates of IL-1β-treated thermosensitive neurons in the VSA by using extracellular microelectrode technique.
, http://www.100md.com
MATERIALS AND METHODS
Surgical procedure The experiments were performed on 26 New Zealand white rabbits of both sexes weighing 1.5-3.0 kg. The animals were anesthetized with 25% urethane (1.0 g/kg,iv). Supplemental doses of anesthetics were given when required. A midline incision was made on the ventral surface of the neck. The animal breathed spontaneously through a trachea tube and was fixed on a stereotaxic frame (Model IIc Jiangwan). Tubular thermodes (Φ1.2 mm) were placed in A0、A5 beyond midline in skull below. Tissue temperature was monitored by a thermistor probe (Φ1.2 mm) placed in a contralateral position symmetrical to that of the recording electrode. Stimulating electrode was insected into the POAH (Stereotaxic coordinates: A2.5、R2.5、H1) , guide cannulae were stereotaxically implanted into the lateral ventricle of rabbits (stereotaxic coordinates: P4.5、R4.0、H6.5). The lateral ventricle infusion was controlled by a microinjector (Shanghai). the environmental temperature was maintained at (22±2)℃during experiment,colon temperature was maintained at 37-39℃.
, http://www.100md.com
Electrophysiological recording Single-Unit recording was made with a glass microelectrode (<1 μm tip, DC resistance 8-15 MΩ, filled with 2% pontamine sky blue and acetate sodium 0.5 mol/L at pH 7.3-7.4. The microelectrode was advanced at the speed of 1μm/s by a micromanipulator (SM-21, Narishige) into the VSA (stereotaxic coordinates: A3-5, R0-3, H0-4) until the activity of an extracellular single unit was recorded. Singals were amplified and filtered by a microelectrode amplifier(MEZ-8300, Nihon Kohden). The amplified electrical signals was monitored on an oscilloscope and recorded by a tape recorder (Victor Company of Japan). Spikes were counted by a computer controled Physiological Signal Processing System(NSA-II, NanJing) and displayed in array density histograms.
, 百拇医药
A monopolar stimulating electrode was constructed from a stainless steel in sect pin (size=00 with a tipe exposure of 500 μm), with the use of a stimulator set to generate monophasic square-wave pulses of 0.5 ms duration, each site was stimulated electrically at a frequency of 50 Hz(30 s, 400 μA).
Experimental protocols After unit-signal activity stabilized 10 minutes,the thermal-response pattern of a unit in H0-4 was determined by recording the units firing rate as brain temperature was slowly raried twice at a rate of about 0.1-2℃/min by super constant temperature pump from about 34℃ to 42℃, neurons were classfied as warm-sensitive if they had a positive thermal coefficient of at least 0.8 impulse/(s*℃) [imps/(s*℃)] or cold-sensitive if they had a thermal coefficient of -0.6 imps/(s*℃) or less. IL-1β(10 mg/L) (BangDin Company of Beijing, China) was injected (icv). After the icv injection of ACSF 10 minutes, effects of electrical stimulation of POAH on the firing rate of neurons in the VSA were recorded.
, 百拇医药
Histological assay At the end of experiment, the dye filled in glass micropippets was diffused out with a negative direct current (20 μA). While the animals were deeply anesthetized, the brain was quickly removed and fixed in 10% formalin. Forzen brain tissue was sectioned in the coronal plane (20-40 μm) after 5-7 d, Histological verification was carried out with reference to Sawyers coordinates[2]. Data from those electrode tips not in the desired area were excluded.
, http://www.100md.com
Statistics Data were expressed as
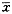 ±s and analyzed using paired Students t test. Differences were considered statistically significant as P<0.05.
±s and analyzed using paired Students t test. Differences were considered statistically significant as P<0.05.RESULTS
Effects of IL-1β on the firing rate of warm-sensitive neurons in the VSA In 14 rabbits, eight spontaneously active single-units in desired area were recorded. After icv injection of IL-1β, the firing rate of warm-sensitive neurons were increased from (5.76±2.58) imps/s (before injection) to (5.80±2.10) imps/s(P>0.05),(8.92±3.73) imps/s(P<0.01), (13.58±6.37) imps/s(P<0.01) and (19.86±6.36) imps/s (P<0.01) at 1, 3,5 and 10 min after icv injection, respectively. However, the firing rate of warm-sensitive neurons had no significant changes (P<0.05) after icv injection of equivalent artificial cerebrospinal fluid (ACSF) (Fig 1). The results indicated that the firing rate of warm-sensitive neurons in the VSA increased remarkably when icv exposed to IL-1β.
, http://www.100md.com
Effects of electrical stimulation of POAH on the firing rate of IL-1β-treated warm-sensitive neurons in the VSA In 14 rabbits, eight spontaneously active single-units were recorded. After icv injection of IL-1β, the firing rate of warm-sensitive neurons increased remarkably. When the POAH was stimulated with current (400 μA), the firing rate of warm-sensitive neurons were decreased from (19.86±10.36) imps/s (before stimulation) to (7.67±5.74) imps/s (P<0.01), (4.19±2.18) imps/s (P<0.01), (1.37±0.36) imps/s (P<0.01), (3.34±2.43) imps/s (P<0.01) and (5.92±2.63) imps/s (P<0.01) at 1, 3, 5, 10 and 15 min after stimulation, respectively (Fig 1).

, 百拇医药
Fig 1 Effects of electrical stimulation POAH on discharge activity of pyrogen (IL-1β)-treated warm-sensitive neurons in VSA (spike array density).
A. spontaneous discharge; B. i.c.v. injection of ACSF;
C. i.c.v. injection of IL-1β; D. electrical stimulation POAH;
E. recover spontaneous discharge.
Effects of IL-1β on the firing rate of cold-sensitive neurons in the VSA In 12 rabbits, six spontaneously active single-units in desired area were recorded. After icv injection of IL-1β, the firing rate of cold-sensitive neurons were decreased from (2.45±0.38) imps/s (before injection) to (2.40±0.53) imps/s (P>0.05),(1.46±0.66) imps/s (P<0.05),(0.89±0.57) imps/s (P<0.01) and (0.49±0.40) imps/s (P<0.01) at 1, 3, 5 and 10 min after icv injection, respectively. However, The firing rate of cold-sensitive neurons had no significant changes (P<0.05) after icv injection of equivalent ACSF (Fig.2). The results indicated that the firing rate of cold-sensitive neurons in the VSA decreased remarkably when icv injection of IL-1β.
, http://www.100md.com
Effects of electrical stimulation of POAH on the firing rate of IL-1β-treated cold-sensitive neurons in the VSA In 12 rabbits, six spontaneously active single-unit in desired area were recorded. After icv injection of IL-1β, the firing rate of cold-sensitive neurons decreased remarkably. When the POAH was stimulated with current (400 μA), the firing rate of cold-sensitive neurons increased from (0.49±0.40) imps/s (before stimulation) to (5.53±4.42) imps/s (P<0.05), (8.9±3.80) imps/s (P<0.05),(18.88±0.21) imps/s (P<0.01)、(7.79±4.26) imps/s (P<0.01) and (2.91±0.62) imps/s (P<0.01) at 1, 3, 5, 10 and 15 min after POAH stimulation, respectively (Fig.2).

, 百拇医药
Fig 2 Effects of electrical stimulation POAH on discharge activity of pyrogen (IL-1β)-treated cold-sensitive neurons in the VSA (spike array density).
A. spontaneous discharge; B. i.c.v. injection of ACSF;
C. i.c.v. injection of IL-1β;
D. electrical stimulation of POAH;
E. recover spontaneous discharge.
, http://www.100md.com DISCUSSION
Considerable evidences have accumulated indicating that endogenous pyrogens (IL-1, TNF, IFN-γ、IL-6 etc) and central febrile mediators (PGE, cAMP, etc.) act principally on the central region of POAH (considered as positive thermoregulatory center) while endogenous antipyretics (AVP, α-MSH, etc.) act primary on the VSA and medial amygdaloid nucleus (considered as negative thermoregulatory center)[2-6]. The altitude of body temperature elevating during pyrogen induced fever results from the interaction of positive and negative regulation[7-9]. An early study with dissect construction had demonstrated that the locus of the POAH and VSA have thermosensitive neurons and widely fiber connections[10]. However, it remains to be elucidated whether this has some functional connection to fever mechanism. Our recent observation found that the firing rate of warm-sensitive neurons decreased and that of cold-sensitive neurons increased remarkably after icv injection of IL-1β. The effect of IL-1β on the thermosensitive neurons in the POAH was reversed by electrical stimulation of the VSA. These findings support the concept that positive and negative thermoregulatory centers are functionally connected. The results also indicate that the VSA plays a negative central role in response to the pyrogen. In the present investigation, we further confirmed that icv of IL-1β affected electrophysiological activity of thermosensitive neurons in the VSA, and electrical stimulation of POAH reversed the effect of IL-1β, indicating that the POAH plays a positive regulatory role. Collectively, these in vivo data reinforced the conclusion: (1) There are at least two types thermoregulatory positive and negative centers involved in fever mechanism. (2) The POAH and VSA are functionally connected.
, 百拇医药
There have been several attempts to study thermoregulation at level of single neuron. Recording firing rate reveals the presence of specialized cells that are very sensitive to imposed changes in local temperature. Our data showed that IL-1β diminished warm-sensitive neuron activity and enhanced cold-sensitive neuron in the POAH, suggesting that POAH is positive in response to the action of endogenous pyrogen and triggers the increase in heat production and decrease in heat lost. Furthermore, electrical stimulation of the VSA reversed the effect of IL-1β, revealing that the VSA has a negative functional connection to the thermosensitive neural activity in the POAH. The data offered in the present investigation further confirm this conclusion and strongly support the view point that the VSA is a negative thermoregulatory center for pyrogen-induced fever. Electrical stimulation of POAH reversed the effect of IL-1β on the neural activity in the VSA, gives a strong evidence that POAH has a positive functional connection to the VSA. Although it is still a question whether IL-1β has direct effect on thermosensitive neurons or connection by neural fibers between the POAH and the VSA remains to be elucidated, it is important that the functional relationship between these two regions has been electrical stitrophysiologically established for explaining fever mechanism.
, 百拇医药
[Foundation item] Work supported by the National Natural Science Foundation of China (No. 39600061); The Office of the Overseas Chinese Affairs of the State Council Scientific Research Foundation (No. 93-95-8)
△ To whom correspondence should be addressed
Tel: 020-85220253
[REFERENCES]
[1] Dong J,Li CJ, Lu DX, et al. Effects of electrical stimulation VSA on the firing of pyrogen-treated thermosensitive neurons in the region of POAH in rabbits[J]. Chin J Pathphysiol, 2000, 16(4): 301-303.
, 百拇医药
[2] Sawyer CH, Everett JW, Green JD. The rabbit diencephalons in stereotaxic coordinates[J]. J Comp Neurol, 1954, 101(2): 801-824.
[3] Federico P, Veale WL, Pittman QJ. Vasopressin-induced antipyresis in the medial amygdaloid nucleus of conscious rats[J]. Am J Physiol, 1992, 262(5Pt2): R901-908.
[4] Federico P, Malkinson TJ, Cooper KE, et al. Vasopressin perfusion within the medial amygdaloid nucleus attenuates prostaglandin fever in the urethane-anaesthetized rat[J]. Brain Res, 1992, 587(2):319-326.
, http://www.100md.com
[5] Yang YL, Wang XM, Shi SG,et al. The effect of microinjection of AVP and AVPMcAb into medial amygdaloid nucleus on endotoxin-induced fever in rabbits[J]. Chin J Appl Physiol, 1999, 15(1):62-65. (in Chinese with English abstract).
[6] Willian D, Ruwe. Electrical Stimulation of the paraventricular nucleus attenuates pyrogen fever in rabbit[J]. Brain Res, 1992, 588:181-190.
[7] Li CJ. Positive-negitive thermoregulation in fever[J]. Chin J Pathophysiol, 1994, 10(5):553-557.
, http://www.100md.com
[8] Zeisberger E, Roth J. Neurobiological concepts of fever generation and suppression[J]. Neuropsychobiology, 1993, 28(1-2):106-109.
[9] Cooper KE, Malkinson TJ, Monda M, et al. New neurobiogical concepts of the genesis of fever and of antipyresis[M]. In: Barfai I, Ottoson D eds. Neuroimmunology of Fever. New York: Pergaman Press, 1992. 225-234.
[10] Cheng ZX. Connection with nucleus and fiber in hypothalamus. Journal of Tianjing Medical College, 1983, 19(1):19-29.
[Received]2000-05-23 [Accepted] 2000-06-22, 百拇医药