脂质体与蛋白质共价偶联的碳二亚胺法
作者:罗招凡 刘剑雄 范 侠$2:%{8|, 百拇医药
单位:罗招凡、范侠(中山医科大学孙逸仙纪念医院检验科 广州 510120);刘剑雄(湖北医科大学第一附属医院检验科)$2:%{8|, 百拇医药
关键词:脂质体;碳二亚胺法$2:%{8|, 百拇医药
免疫学杂志990217 摘 要 报道脂质体与蛋白类物质胰岛素共价偶联的碳二亚胺(EDCI)法,取一定量脂质体、EDCI及胰岛素液,pH7.4、24°C条件下充分反应2h,用低温高速离心使脂质体-胰岛素复合物与游离胰岛素分开,并将本法与戊二醛法作了比较。结果:碳二亚胺首先与胰岛素上的羧基反应生成一加成中间产物,然后再与脂质体上的氨基反应生成酰胺键,实现两者的交联。EDCI法高效、方便,是一种较好的将蛋白类物质偶联于脂质体上的化学方法。$2:%{8|, 百拇医药
中图号 R392-33$2:%{8|, 百拇医药
CONVALENT ATTACHMENT OF PROTEIN TO$2:%{8|, 百拇医药
LIPOSOMES BY EDCI METHOD$2:%{8|, 百拇医药
Luo Zhaofan,Liu Jianxiong,Fan Xia$2:%{8|, 百拇医药
(Department of Laboratory Science,The Sun Yat-Sen Memorial$2:%{8|, 百拇医药
Hospital,Guangzhou 510120)$2:%{8|, 百拇医药
Abstract This paper introduced a method for convalent binding of protein such as insulin to liposome by 1-ethy1-3-(3-dimethylaminopropyl) carbodiimid(EDCI).In the condition of pH7.4,a mixture solution of EDCI and insulin was added to 0.3ml liposomes,after standing for 2h at 24°C,free insulin could be separated from liposome-insulin compounds through ultracentrifugation.The comparison between the EDCI and glutaraldehytede methods showed that the EDCI method is efficient and convenient for the chemical modification of liposomes with proteins.
Key words Liposome,EDCI method/fu, 百拇医药
脂质体与蛋白质偶联获得免疫脂质体,建立脂质体免疫试验(Liposome Immunoassay,LIA)[1]为临床相关物质的检测提供了一条新途径,具有较高的实用价值[2],国内目前尚未见此方面的报道。本文对HASHIMOTO[3]介绍的碳二亚胺法进行了改进,并与经典的戊二醛标记法[4]作了比较。/fu, 百拇医药
1 材料与方法/fu, 百拇医药
1.1 材料与仪器 二软脂酰磷酯酰胆碱(Di-palmitoylphosphatidylcholine,DPPC),二软脂酰磷酯酰乙醇胺(DPPE)、胆固醇(Cholesterol,CHOL)、丽丝胺罗丹明B(Sulforhodamine B SRB)、乙基替-(3-二甲氨基丙基)碳二亚胺(EDCI)均为进口原装SIGMA试剂,胰岛素干粉(批号 950302,徐州生物化学制药厂生产),上海产ZFQ85A型旋转蒸发器,通化产JC-3型超声处理机,日立H-600型透射电镜,BECKMAN高速离心机,上海产SN-682型放射免疫γ计数器。/fu, 百拇医药
1.2 脂质体的制备 将DPPC、CHOL、DPPE按一定比例制成3种脂质体。其中小单层脂质体(Small Unilamellar Vesicles,SUVs)采用直接超声法[5],大单层脂质体(Large Unilamellar Vesicles,LUVs)采用逆相蒸发法[6],多层脂质体(Multilamellar Vesicles,MLVs)采用振摇法[7]。/fu, 百拇医药
1.3 脂质体与胰岛素的偶联 采用EDCI法[3],方法稍加改进。实验分两组,一组为加EDCI组,另一组不加EDCI。取3种脂质体0.3ml于实验各管,第一组各管内分别加入0.4ml的20%EDCI液(溶于pH7.4 Gelatin veronal buffered saline,GVB)和4ml 1mg/ml,0.5mg/ml,0.25mg/ml,0.125mg/ml,0.0625mg/ml胰岛素液(溶于pH7.4 GVB液)的混合反应液,第二组EDCI液用等量GVB 液取代。混合各管后,于24°C静置2h,12 000×g,4°C离心15min。弃上清,用GVB液重复洗涤离心5次,弃上清,最后将各管沉淀脂质体重悬于0.3mlGVB液,置4°C冰箱备用。
1.4 指标测定 于上述各管各取10μ1脂质体液稀释104倍,放射免疫法(RIA)测定脂质体上偶联胰岛素浓度,空白管以GVB液取代。sx3/-$, http://www.100md.com
2 结果sx3/-$, http://www.100md.com
2.1 电镜下形态 经电镜观察:SUVs直径为30±5nm,呈均匀圆形;LUVs直径420±30nm,较均一;MLVs直径大于1 000nm。见图1,2,3。
 sx3/-$, http://www.100md.com
sx3/-$, http://www.100md.com
图 1 SUVs负染电镜照片(×40 000)sx3/-$, http://www.100md.com
Fig 1 Negative stain electron micrograph of SUVssx3/-$, http://www.100md.com
图 2 LUVs负染电镜照片(×30 000)sx3/-$, http://www.100md.com
Fig 2 Negative stain electron micrograph of LUVs sx3/-$, http://www.100md.com
sx3/-$, http://www.100md.com
图 3 MLVs负染电镜照片(×20 000)sx3/-$, http://www.100md.com
Fig 3 Negative stain electron micrograph of MLVssx3/-$, http://www.100md.com
2.2 脂质体偶联胰岛素结果 在同一条件下,脂质体(DPPC∶CHOL∶DPPE=36∶33∶6)上偶联胰岛素浓度EDCI组明显高于无EDCI组,且随着胰岛素初始浓度的升高而升高。见图4。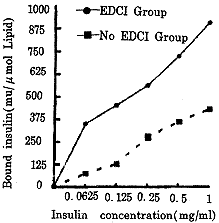 sx3/-$, http://www.100md.com
sx3/-$, http://www.100md.com
图 4 RIA检测EDCI组和无EDCI组脂质体偶联胰岛素结果sx3/-$, http://www.100md.com
Fig 4 Results of insulin covalent binding LUVs in EDCI and no EDCI group by RIAsx3/-$, http://www.100md.com
2.3 不同脂质体的标记效率比较 在相同条件下,标记在SUVs上的蛋白质与其脂的比率最高,其次是LUVs,最后是MLVs。见表1。|ho, http://www.100md.com
表1 LUVs MLVs和SUVs标记胰岛素效率比较|ho, http://www.100md.com
Tab 1 Comparison of insulin labelling efficiency among LUVs MIVs和SUVs Initial protein|ho, http://www.100md.com
concentration(mg/ml)|ho, http://www.100md.com
Types of|ho, http://www.100md.com
liposomes|ho, http://www.100md.com
Protein/lipid|ho, http://www.100md.com
(102g/mol)|ho, http://www.100md.com
Efficincy|ho, http://www.100md.com
%|ho, http://www.100md.com
bound protein|ho, http://www.100md.com
1|ho, http://www.100md.com
LUVs|ho, http://www.100md.com
3.24|ho, http://www.100md.com
3.24|ho, http://www.100md.com
2.43|ho, http://www.100md.com
MLVs|ho, http://www.100md.com
2.11|ho, http://www.100md.com
2.11|ho, http://www.100md.com
1.58|ho, http://www.100md.com
SUVs|ho, http://www.100md.com
4.31|ho, http://www.100md.com
4.31|ho, http://www.100md.com
3.23|ho, http://www.100md.com
0.5
LUVs?ikv&, 百拇医药
2.59?ikv&, 百拇医药
5.18?ikv&, 百拇医药
3.89?ikv&, 百拇医药
MLVs?ikv&, 百拇医药
1.31?ikv&, 百拇医药
2.62?ikv&, 百拇医药
1.97?ikv&, 百拇医药
SUVs?ikv&, 百拇医药
3.37?ikv&, 百拇医药
6.74?ikv&, 百拇医药
5.06?ikv&, 百拇医药
2.4 二软脂酰磷脂酰乙醇胺(DPPE)含量对胰岛素标记效率的影响 当总脂含量一定时,改变DPPE对总脂的比例,则影响偶联的所有参数。见表2。表2 LUVs中不同比例DPPE标记胰岛素效率的比较?ikv&, 百拇医药
Tab 2 Comparison of insulin labbelling with different ratios of DPPE in LUVs Initial protein?ikv&, 百拇医药
(mg/ml)?ikv&, 百拇医药
Lipids com-?ikv&, 百拇医药
position(DP∶?ikv&, 百拇医药
PC∶CHOL∶DPPE)?ikv&, 百拇医药
Protein∶Lipid?ikv&, 百拇医药
(102g/mol)?ikv&, 百拇医药
Efficiency?ikv&, 百拇医药
%?ikv&, 百拇医药
bound protein?ikv&, 百拇医药
1?ikv&, 百拇医药
36∶33∶6\dzt, http://www.100md.com
3.24\dzt, http://www.100md.com
3.24\dzt, http://www.100md.com
2.43\dzt, http://www.100md.com
36∶33∶3\dzt, http://www.100md.com
1.91\dzt, http://www.100md.com
1.91\dzt, http://www.100md.com
1.43\dzt, http://www.100md.com
36∶33∶6\dzt, http://www.100md.com
2.59\dzt, http://www.100md.com
5.18\dzt, http://www.100md.com
3.89\dzt, http://www.100md.com
0.5\dzt, http://www.100md.com
36∶33∶4.5\dzt, http://www.100md.com
2.07\dzt, http://www.100md.com
4.14\dzt, http://www.100md.com
3.10\dzt, http://www.100md.com
2.5 碳二亚胺法与戊二醛法比较 见表3。表3 碳二亚胺法与戊二醛法标记胰岛素效率比较\dzt, http://www.100md.com
Tab 3 Comparison between methods of EDCI and glutaraldehyde Method\dzt, http://www.100md.com
Initial pro-\dzt, http://www.100md.com
tein(mg/ml)\dzt, http://www.100md.com
Lipid composi-\dzt, http://www.100md.com
tion (DPP∶\dzt, http://www.100md.com
CHOL∶DPPE)\dzt, http://www.100md.com
Protein:Lipid\dzt, http://www.100md.com
(102g/mol)
Efficiency4, http://www.100md.com
%bound4, http://www.100md.com
protein4, http://www.100md.com
Glutaral-4, http://www.100md.com
dehyde4, http://www.100md.com
14, http://www.100md.com
0.54, http://www.100md.com
5∶4∶14, http://www.100md.com
5∶4∶14, http://www.100md.com
4.324, http://www.100md.com
2.514, http://www.100md.com
4.324, http://www.100md.com
5.024, http://www.100md.com
3.034, http://www.100md.com
3.514, http://www.100md.com
EDCI4, http://www.100md.com
14, http://www.100md.com
0.54, http://www.100md.com
36∶33∶124, http://www.100md.com
36∶33∶124, http://www.100md.com
3.244, http://www.100md.com
2.594, http://www.100md.com
3.244, http://www.100md.com
5.184, http://www.100md.com
2.434, http://www.100md.com
3.894, http://www.100md.com
All lipsomes are LUV.Glutaraldehyde Method was refered to Torchilin VP[4].4, http://www.100md.com
3 讨论
本文采用水溶性的碳二亚胺将胰岛素偶联于脂质体上,整个实验条件温和。从图4结果可知:免疫脂质体上偶联胰岛素浓度不仅与有无EDCI有关,而且还与胰岛素的初始浓度有关,这一线性关系保证了脂质体上要偶联一定量胰岛素可通过控制胰岛素的初始浓度来实现。此外,为了尽量减少胰岛素间的自身聚合,可先将胰岛素和EDCI反应,活化羧基后再加入脂质体。实验有所改进之处就是选择以GVB液为洗液,多次、反复于12 000×g 4°C高速离心,而不采用原文报道的以Ficoll-paque细胞分离液750×g离心,其目的是为了彻底去除游离的胰岛素,且不损失脂质体。&:q^%[, http://www.100md.com
本文对LUVs、MLVs、SUVs3种脂质体进行了标记,结果与电镜观察的结构基本符合。MLV具多层结构,部分脂在内部无法得到标记,所以标记率约为LUV的一半,而SUV脂双层的脂质分布不均匀,外层脂占总脂的60%~70%[8],使它的标记率最高。这也提示了脂质体在标记过程中形态改变不大。&:q^%[, http://www.100md.com
在适当条件下,碳二亚胺法与戊二醛法标记率相当,但后者还需特殊试剂如硼氢化钠等,方法较繁,且难以避免脂质体之间、抗体之间的自身交联,使得产物均一性较差。&:q^%[, http://www.100md.com
通过EDCI法获得免疫脂质体的实验,为建立体外的脂质体免疫实验(LIA)提供了实验基础。&:q^%[, http://www.100md.com
作者简介 第一作者:女,26岁,硕士,检验师&:q^%[, http://www.100md.com
参考文献&:q^%[, http://www.100md.com
1 Rongen HAH,Bult A,Van Bennekon WP.Liposomes and immunoassays (Review),Immunol Methods,1997,204:105&:q^%[, http://www.100md.com
2 李金明.脂质体免疫测定技术.《国外医学》临床生物化学与检验分册,1993,14(3):116&:q^%[, http://www.100md.com
3 Hashimoto Y,Endoh H,Sugawara M.Chemical method for the modification of lipsomes with proteins or antibodise.In:Gregoriadis G.ed.Liposome Technology.Vol3.Florida:CRC press,1984.44&:q^%[, http://www.100md.com
4 Torchilin VP,Goldmacher VS,Smirnov VN.Preservation of antimyosin antibody activity after covalent coupling to liposomes.Biochem Biophys Res Commun,1978,85:983&:q^%[, http://www.100md.com
5 Johnson SM,Bangham AD,Hill MW,et al.Single bilayer liposomes.Biochem Biophys Acta.1971,233:820&:q^%[, http://www.100md.com
6 Szoka F,JR,Papahadjopoulos D.Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation.Proc Natl Acad Sci USA,1978,75(9):4194&:q^%[, http://www.100md.com
7 Olson F,Hunt CA,Szkoka F,et al.Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes.Biochem Biophys Acta,1979,557(1):9&:q^%[, http://www.100md.com
8 周之鸿,徐宁宁,陈庆才,等.脂质体中磷脂的含磷量测定.药学通报,1987,2:77&:q^%[, http://www.100md.com
(1998-10-19收稿;1998-11-30修回)(罗招凡 刘剑雄 范 侠)
单位:罗招凡、范侠(中山医科大学孙逸仙纪念医院检验科 广州 510120);刘剑雄(湖北医科大学第一附属医院检验科)$2:%{8|, 百拇医药
关键词:脂质体;碳二亚胺法$2:%{8|, 百拇医药
免疫学杂志990217 摘 要 报道脂质体与蛋白类物质胰岛素共价偶联的碳二亚胺(EDCI)法,取一定量脂质体、EDCI及胰岛素液,pH7.4、24°C条件下充分反应2h,用低温高速离心使脂质体-胰岛素复合物与游离胰岛素分开,并将本法与戊二醛法作了比较。结果:碳二亚胺首先与胰岛素上的羧基反应生成一加成中间产物,然后再与脂质体上的氨基反应生成酰胺键,实现两者的交联。EDCI法高效、方便,是一种较好的将蛋白类物质偶联于脂质体上的化学方法。$2:%{8|, 百拇医药
中图号 R392-33$2:%{8|, 百拇医药
CONVALENT ATTACHMENT OF PROTEIN TO$2:%{8|, 百拇医药
LIPOSOMES BY EDCI METHOD$2:%{8|, 百拇医药
Luo Zhaofan,Liu Jianxiong,Fan Xia$2:%{8|, 百拇医药
(Department of Laboratory Science,The Sun Yat-Sen Memorial$2:%{8|, 百拇医药
Hospital,Guangzhou 510120)$2:%{8|, 百拇医药
Abstract This paper introduced a method for convalent binding of protein such as insulin to liposome by 1-ethy1-3-(3-dimethylaminopropyl) carbodiimid(EDCI).In the condition of pH7.4,a mixture solution of EDCI and insulin was added to 0.3ml liposomes,after standing for 2h at 24°C,free insulin could be separated from liposome-insulin compounds through ultracentrifugation.The comparison between the EDCI and glutaraldehytede methods showed that the EDCI method is efficient and convenient for the chemical modification of liposomes with proteins.
Key words Liposome,EDCI method/fu, 百拇医药
脂质体与蛋白质偶联获得免疫脂质体,建立脂质体免疫试验(Liposome Immunoassay,LIA)[1]为临床相关物质的检测提供了一条新途径,具有较高的实用价值[2],国内目前尚未见此方面的报道。本文对HASHIMOTO[3]介绍的碳二亚胺法进行了改进,并与经典的戊二醛标记法[4]作了比较。/fu, 百拇医药
1 材料与方法/fu, 百拇医药
1.1 材料与仪器 二软脂酰磷酯酰胆碱(Di-palmitoylphosphatidylcholine,DPPC),二软脂酰磷酯酰乙醇胺(DPPE)、胆固醇(Cholesterol,CHOL)、丽丝胺罗丹明B(Sulforhodamine B SRB)、乙基替-(3-二甲氨基丙基)碳二亚胺(EDCI)均为进口原装SIGMA试剂,胰岛素干粉(批号 950302,徐州生物化学制药厂生产),上海产ZFQ85A型旋转蒸发器,通化产JC-3型超声处理机,日立H-600型透射电镜,BECKMAN高速离心机,上海产SN-682型放射免疫γ计数器。/fu, 百拇医药
1.2 脂质体的制备 将DPPC、CHOL、DPPE按一定比例制成3种脂质体。其中小单层脂质体(Small Unilamellar Vesicles,SUVs)采用直接超声法[5],大单层脂质体(Large Unilamellar Vesicles,LUVs)采用逆相蒸发法[6],多层脂质体(Multilamellar Vesicles,MLVs)采用振摇法[7]。/fu, 百拇医药
1.3 脂质体与胰岛素的偶联 采用EDCI法[3],方法稍加改进。实验分两组,一组为加EDCI组,另一组不加EDCI。取3种脂质体0.3ml于实验各管,第一组各管内分别加入0.4ml的20%EDCI液(溶于pH7.4 Gelatin veronal buffered saline,GVB)和4ml 1mg/ml,0.5mg/ml,0.25mg/ml,0.125mg/ml,0.0625mg/ml胰岛素液(溶于pH7.4 GVB液)的混合反应液,第二组EDCI液用等量GVB 液取代。混合各管后,于24°C静置2h,12 000×g,4°C离心15min。弃上清,用GVB液重复洗涤离心5次,弃上清,最后将各管沉淀脂质体重悬于0.3mlGVB液,置4°C冰箱备用。
1.4 指标测定 于上述各管各取10μ1脂质体液稀释104倍,放射免疫法(RIA)测定脂质体上偶联胰岛素浓度,空白管以GVB液取代。sx3/-$, http://www.100md.com
2 结果sx3/-$, http://www.100md.com
2.1 电镜下形态 经电镜观察:SUVs直径为30±5nm,呈均匀圆形;LUVs直径420±30nm,较均一;MLVs直径大于1 000nm。见图1,2,3。

 sx3/-$, http://www.100md.com
sx3/-$, http://www.100md.com图 1 SUVs负染电镜照片(×40 000)sx3/-$, http://www.100md.com
Fig 1 Negative stain electron micrograph of SUVssx3/-$, http://www.100md.com
图 2 LUVs负染电镜照片(×30 000)sx3/-$, http://www.100md.com
Fig 2 Negative stain electron micrograph of LUVs
 sx3/-$, http://www.100md.com
sx3/-$, http://www.100md.com图 3 MLVs负染电镜照片(×20 000)sx3/-$, http://www.100md.com
Fig 3 Negative stain electron micrograph of MLVssx3/-$, http://www.100md.com
2.2 脂质体偶联胰岛素结果 在同一条件下,脂质体(DPPC∶CHOL∶DPPE=36∶33∶6)上偶联胰岛素浓度EDCI组明显高于无EDCI组,且随着胰岛素初始浓度的升高而升高。见图4。
 sx3/-$, http://www.100md.com
sx3/-$, http://www.100md.com图 4 RIA检测EDCI组和无EDCI组脂质体偶联胰岛素结果sx3/-$, http://www.100md.com
Fig 4 Results of insulin covalent binding LUVs in EDCI and no EDCI group by RIAsx3/-$, http://www.100md.com
2.3 不同脂质体的标记效率比较 在相同条件下,标记在SUVs上的蛋白质与其脂的比率最高,其次是LUVs,最后是MLVs。见表1。|ho, http://www.100md.com
表1 LUVs MLVs和SUVs标记胰岛素效率比较|ho, http://www.100md.com
Tab 1 Comparison of insulin labelling efficiency among LUVs MIVs和SUVs Initial protein|ho, http://www.100md.com
concentration(mg/ml)|ho, http://www.100md.com
Types of|ho, http://www.100md.com
liposomes|ho, http://www.100md.com
Protein/lipid|ho, http://www.100md.com
(102g/mol)|ho, http://www.100md.com
Efficincy|ho, http://www.100md.com
%|ho, http://www.100md.com
bound protein|ho, http://www.100md.com
1|ho, http://www.100md.com
LUVs|ho, http://www.100md.com
3.24|ho, http://www.100md.com
3.24|ho, http://www.100md.com
2.43|ho, http://www.100md.com
MLVs|ho, http://www.100md.com
2.11|ho, http://www.100md.com
2.11|ho, http://www.100md.com
1.58|ho, http://www.100md.com
SUVs|ho, http://www.100md.com
4.31|ho, http://www.100md.com
4.31|ho, http://www.100md.com
3.23|ho, http://www.100md.com
0.5
LUVs?ikv&, 百拇医药
2.59?ikv&, 百拇医药
5.18?ikv&, 百拇医药
3.89?ikv&, 百拇医药
MLVs?ikv&, 百拇医药
1.31?ikv&, 百拇医药
2.62?ikv&, 百拇医药
1.97?ikv&, 百拇医药
SUVs?ikv&, 百拇医药
3.37?ikv&, 百拇医药
6.74?ikv&, 百拇医药
5.06?ikv&, 百拇医药
2.4 二软脂酰磷脂酰乙醇胺(DPPE)含量对胰岛素标记效率的影响 当总脂含量一定时,改变DPPE对总脂的比例,则影响偶联的所有参数。见表2。表2 LUVs中不同比例DPPE标记胰岛素效率的比较?ikv&, 百拇医药
Tab 2 Comparison of insulin labbelling with different ratios of DPPE in LUVs Initial protein?ikv&, 百拇医药
(mg/ml)?ikv&, 百拇医药
Lipids com-?ikv&, 百拇医药
position(DP∶?ikv&, 百拇医药
PC∶CHOL∶DPPE)?ikv&, 百拇医药
Protein∶Lipid?ikv&, 百拇医药
(102g/mol)?ikv&, 百拇医药
Efficiency?ikv&, 百拇医药
%?ikv&, 百拇医药
bound protein?ikv&, 百拇医药
1?ikv&, 百拇医药
36∶33∶6\dzt, http://www.100md.com
3.24\dzt, http://www.100md.com
3.24\dzt, http://www.100md.com
2.43\dzt, http://www.100md.com
36∶33∶3\dzt, http://www.100md.com
1.91\dzt, http://www.100md.com
1.91\dzt, http://www.100md.com
1.43\dzt, http://www.100md.com
36∶33∶6\dzt, http://www.100md.com
2.59\dzt, http://www.100md.com
5.18\dzt, http://www.100md.com
3.89\dzt, http://www.100md.com
0.5\dzt, http://www.100md.com
36∶33∶4.5\dzt, http://www.100md.com
2.07\dzt, http://www.100md.com
4.14\dzt, http://www.100md.com
3.10\dzt, http://www.100md.com
2.5 碳二亚胺法与戊二醛法比较 见表3。表3 碳二亚胺法与戊二醛法标记胰岛素效率比较\dzt, http://www.100md.com
Tab 3 Comparison between methods of EDCI and glutaraldehyde Method\dzt, http://www.100md.com
Initial pro-\dzt, http://www.100md.com
tein(mg/ml)\dzt, http://www.100md.com
Lipid composi-\dzt, http://www.100md.com
tion (DPP∶\dzt, http://www.100md.com
CHOL∶DPPE)\dzt, http://www.100md.com
Protein:Lipid\dzt, http://www.100md.com
(102g/mol)
Efficiency4, http://www.100md.com
%bound4, http://www.100md.com
protein4, http://www.100md.com
Glutaral-4, http://www.100md.com
dehyde4, http://www.100md.com
14, http://www.100md.com
0.54, http://www.100md.com
5∶4∶14, http://www.100md.com
5∶4∶14, http://www.100md.com
4.324, http://www.100md.com
2.514, http://www.100md.com
4.324, http://www.100md.com
5.024, http://www.100md.com
3.034, http://www.100md.com
3.514, http://www.100md.com
EDCI4, http://www.100md.com
14, http://www.100md.com
0.54, http://www.100md.com
36∶33∶124, http://www.100md.com
36∶33∶124, http://www.100md.com
3.244, http://www.100md.com
2.594, http://www.100md.com
3.244, http://www.100md.com
5.184, http://www.100md.com
2.434, http://www.100md.com
3.894, http://www.100md.com
All lipsomes are LUV.Glutaraldehyde Method was refered to Torchilin VP[4].4, http://www.100md.com
3 讨论
本文采用水溶性的碳二亚胺将胰岛素偶联于脂质体上,整个实验条件温和。从图4结果可知:免疫脂质体上偶联胰岛素浓度不仅与有无EDCI有关,而且还与胰岛素的初始浓度有关,这一线性关系保证了脂质体上要偶联一定量胰岛素可通过控制胰岛素的初始浓度来实现。此外,为了尽量减少胰岛素间的自身聚合,可先将胰岛素和EDCI反应,活化羧基后再加入脂质体。实验有所改进之处就是选择以GVB液为洗液,多次、反复于12 000×g 4°C高速离心,而不采用原文报道的以Ficoll-paque细胞分离液750×g离心,其目的是为了彻底去除游离的胰岛素,且不损失脂质体。&:q^%[, http://www.100md.com
本文对LUVs、MLVs、SUVs3种脂质体进行了标记,结果与电镜观察的结构基本符合。MLV具多层结构,部分脂在内部无法得到标记,所以标记率约为LUV的一半,而SUV脂双层的脂质分布不均匀,外层脂占总脂的60%~70%[8],使它的标记率最高。这也提示了脂质体在标记过程中形态改变不大。&:q^%[, http://www.100md.com
在适当条件下,碳二亚胺法与戊二醛法标记率相当,但后者还需特殊试剂如硼氢化钠等,方法较繁,且难以避免脂质体之间、抗体之间的自身交联,使得产物均一性较差。&:q^%[, http://www.100md.com
通过EDCI法获得免疫脂质体的实验,为建立体外的脂质体免疫实验(LIA)提供了实验基础。&:q^%[, http://www.100md.com
作者简介 第一作者:女,26岁,硕士,检验师&:q^%[, http://www.100md.com
参考文献&:q^%[, http://www.100md.com
1 Rongen HAH,Bult A,Van Bennekon WP.Liposomes and immunoassays (Review),Immunol Methods,1997,204:105&:q^%[, http://www.100md.com
2 李金明.脂质体免疫测定技术.《国外医学》临床生物化学与检验分册,1993,14(3):116&:q^%[, http://www.100md.com
3 Hashimoto Y,Endoh H,Sugawara M.Chemical method for the modification of lipsomes with proteins or antibodise.In:Gregoriadis G.ed.Liposome Technology.Vol3.Florida:CRC press,1984.44&:q^%[, http://www.100md.com
4 Torchilin VP,Goldmacher VS,Smirnov VN.Preservation of antimyosin antibody activity after covalent coupling to liposomes.Biochem Biophys Res Commun,1978,85:983&:q^%[, http://www.100md.com
5 Johnson SM,Bangham AD,Hill MW,et al.Single bilayer liposomes.Biochem Biophys Acta.1971,233:820&:q^%[, http://www.100md.com
6 Szoka F,JR,Papahadjopoulos D.Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation.Proc Natl Acad Sci USA,1978,75(9):4194&:q^%[, http://www.100md.com
7 Olson F,Hunt CA,Szkoka F,et al.Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes.Biochem Biophys Acta,1979,557(1):9&:q^%[, http://www.100md.com
8 周之鸿,徐宁宁,陈庆才,等.脂质体中磷脂的含磷量测定.药学通报,1987,2:77&:q^%[, http://www.100md.com
(1998-10-19收稿;1998-11-30修回)(罗招凡 刘剑雄 范 侠)