STUDY ON PERIPHERAL BLOOD MONONUC-LEAR CELL(PBMC) PROLIFERATIVE RES-PONSES TO DIFFERENT HCV ANTIGENS IN PATIENTS WITH HEPATITIS C*
作者:Dou Jun(窦骏), Liu Kezhou(刘克洲),Chen Zhi(陈智)1, Wo Jian’er(沃建尔),He Nanxiang(何南祥)1,Xu Chenhuai(徐陈槐)1, Zhang Mingtai(章明太)2, Wang Xinzi(王信子)2]n, http://www.100md.com
单位:]n, http://www.100md.com
关键词:丙型肝炎病毒 丙型肝炎病人 外周血单个核细胞 细胞免疫]n, http://www.100md.com
免疫学杂志990102 摘 要 为探讨丙型肝炎(HC)病人细胞免疫功能和丙型肝炎病毒(HCV)的致病机及机体对其免疫保护作用,收集24例HC病人(急性3例,慢性21例),用3H-TdR掺入法研究病人外周血单个核细胞(PBMC)对不同HCV抗原增殖反应,并用流式细胞仪(FACS)检测了PBMC中CD4+、CD8+淋巴细胞亚群在HCV抗原刺激后的变化。结果:HC病人PBMC对HCV合成肽CP9,NS4和基因重组抗原C,E1,E2,NS3刺激后出现不同程度增殖反应,刺激指数(SI)分别为1.69±0.51,1.61±0.54,1.68±0.58,1.49±0.44,1.44±0.44和1.33±0.33。3例急性HC中2例病人的PBMC对HCV抗原呈有效增殖反应(SI≥2.1),且血清HCVRNA阴转伴ALT正常。细胞表型分析显示:增殖的细胞表型是CD4+淋巴细胞,而CD8+淋巴细胞增殖反应较弱。结论:HC病人PBMC确实存在对HCV抗原的增殖反应;CD4+淋巴细胞比CD8+淋巴细胞增殖反应要强,急性HC病人PBMC对HCV抗原有效的增殖反应预示可能有良好的临床愈合。]n, http://www.100md.com
中图号 R374.21]n, http://www.100md.com
Abstract To explore the cellular immune function in patients with hepatitis C and study the HCV pathogenic mechanism and immune protection effect against HCV infection in host. PBMC proliferative responses in vitro to HCV synthetic peptides (Cp9, Ns4) and recombinant HCV proteins (C,E1,E2,NS3) were evaluated in 21 patients with chronic hepatitis C and 3 patients with acute hepatitis C. Phenotypic characteristics of proliferating cells in PBMC were detected by FACS after PBMC were stimulated with HCV antigens. Results showed that lymphoproliferative responses of PBMC from patients with hepatitis C to synthetic peptides (Cp9, NS4) and recombinant proteins (C,E1,E2 and NS3) were detected and its stimulation index(SI) were 1.69±0.51, 1.61±0.54, 1.68±0.58, 1.49±0.44, 1.44±0.44 and 1.33±0.33 respectively. Two out of three patients with acute hepatitis C had efficient lymphoproliferative response to HCV antigens (SI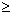 2.1) and their serum HCV-RNA were seroconversion and serum ALT values were returned to normal when studied. Phenotype analysis showed that phenotypic characteristics of proliferating cells was CD4+ lymphocytes and CD8+ lymphocytes proliferative responses was less than that of CD4+ lymphocytes. The results indicate that PBMC from patients with hepatitis C exist proliferative responses to different HCV antigens. CD4+ lymphocytes proliferative response is greater than that of CD8+ lymphocytes. The efficient lymphoproliferative responses to HCV antigens in patients with acute hepatitis C suggests that the patients possibly have a good clinical prognosis.
2.1) and their serum HCV-RNA were seroconversion and serum ALT values were returned to normal when studied. Phenotype analysis showed that phenotypic characteristics of proliferating cells was CD4+ lymphocytes and CD8+ lymphocytes proliferative responses was less than that of CD4+ lymphocytes. The results indicate that PBMC from patients with hepatitis C exist proliferative responses to different HCV antigens. CD4+ lymphocytes proliferative response is greater than that of CD8+ lymphocytes. The efficient lymphoproliferative responses to HCV antigens in patients with acute hepatitis C suggests that the patients possibly have a good clinical prognosis.
Key words Hepatitis C virus,Patients with hepatitis C,Peripheral blood mononuclear cell,Cellular immunet8, http://www.100md.com
Hepatitis C virus (HCV) has been identified as a major etiological agent of parentally and community-acquired NANB hepatitis and most patients with hepatitis C are not able to eliminate the virus[1~2]. However, it is still not known that HCV pathogenic mechanism and immune protecting effect against HCV infection in host at present. The presence of antibodies to most HCV antigens in patients with chronic hepatitis C suggests that the humoral immune responses to the virus is unable to stop the progression of disease[3~8]. In order to explore the cellular immune function in patients with hepatitis C peripheral blood mononuclear cell (PBMC) proliferative res-ponses in vitro to HCV synthetic peptides (Cp9,NS4) and gene recombinant HCV proteins (C,E1,E2,NS3) were evaluated in 21 patients with chronic hepatitis C and 3 patients with acute hepatitis C. We are try to analysis whether there are a relationship between PBMC immune responses in patients with hepatitis C to various HCV antigens and clinical prognosis of HCV infection and to select HCV predominant antigens which are to be thought of having a good immunogenicity and provide the basis for studing the function of PBMC from patients with hepatitis C and HCV vaccine development in future.
MATERIAL AND METHODS9z\6), http://www.100md.com
HCV-seropositive individuals For the present study we selected 24 individuals with serum HCV-RNA positive detected by reverse transcription-nested polymerase chain reaction (RT-nested-PCR). The assay was performed as previously described[9], Briefly, RNA was extracted from 50μl of serum by a modification of a method described elsewhere[10]. After reverse transcription at 42°C for 90 minutes, complementary DNA was amplified by two round PCR by using primers from the core region of HCV genome. (positive sense:5′ G G T C G C A A C C T C G T G G A A G G 3′,negative sense:5′A T C G A T G A C C T T A C C C A A A T 3′). The amplification profile was as follows:denaturation at 95°C for 1 minute, annealing at 55°C for 1 minute and extension at 72°C for 45 seconds, total of 30 cycles. PCR products (209pb) were subjected to electrophoresis in 2% agarose gels, stained with ethidium bremide, and visualized under ultraviolet illumination.9z\6), http://www.100md.com
Patients with chronic hepatitis C This group consisted of 21 patients [17 male and 4 female; median age: 44 years, (range, 27~65)]who had elevated serum levels(>40IU/L)of alanine amino transferase(ALT) for 1 year and liver histology compatible with the diagnosis of chronic hepatitis by the diagnosis criterion of viral hepatitis prevention and treatment in the fifth national symposium on infectious diseases and parastie diseases, May, 1995, Beijing, P.R.China.
Patients with acute hepatitis C This group consisted of 3 individuals [1 male and 2 female,median age: 30 years (range, 25~37)] with a history of blood transfusion, whose serum levels of ALT>40 IU/L for no more than 3 months and whose signs and symptoms were compatible with the dia-gnosis of acute hepatitis by the diagnosis criterion of viral hepatitis prevention and treatment in the fifth national symposium on infectious diseases and parasite diseases, May, 1995, Beijing, P.R.China.o96]li*, http://www.100md.com
HCV-seronegative individuals As controls for the specificity of PBMC responses,we selected 8 healthy individuals without a clinical history of hepatitis and without symptoms or signs of liver disease and serum HCV RNA negative [6 male and 2 female, median age: 29 years (range, 21~41)].o96]li*, http://www.100md.com
HCV antigens HCV recombinant proteins: The HCV proteins core, envelope 1(E1), envelope 2 (E2) nonstructural 3(NS3) were supplied and gifted by prof. Zhuang Hui (Dept. of Microbiology, Beijing Medical University, P.R.China) and Dr.wang Xingtai, Dr. Zhang Shuming (Dept. of hepatitis, national institute for the control of pharmaceutical and biological products. Beijing, P.R.China).
HCV synthetic peptides: The HCV synthetic peptides Cp9,NS4 were syntheted by Institute of Biochemistry Chinese Academy of Medicine, Shanghai, P.R.China).$?{e#%, 百拇医药
Cell Culture-medium The medium used of all cell cultures consisted of RPMI 1640 supplemented with 25 mmol/L HEPES, 2 mmol/L L-glutamine (all from GibcoBRL,Scotland), Penicillin (100u/ml), streptomycin (100μg/ml) and 5% (voL/voL) pooled human serum(RPMI-HS). The AB serum was obtained from healthy blood donors with blood group AB+ and was only used when antibodies to HCV were absent.$?{e#%, 百拇医药
PBMC proliferation assay Heparinized venous blood drawn from patients with hepatitis C were diluted in Hank’s liquid. PBMC were separated by Ficoll-Hypaque (The second agent factory, Shanghai, P.R.China)density gradient centrifugation. Proliferation assays were performed as described [4-5]. Briefly, PBMC(2×105 cells) in 0.2 ml of RPMI-HS were cultured in 96-well flat-bottom microplates in the presence or absence of HCV synthetic peptides or HCV recombinant proteins at various final concentration for 6 days at 37°C in an atmosphere of 5% CO2 in air. 0.5 μCi of [3H] thymidine (3H-TdR,Purchased from Institute of radionuelide, Chinese Academy of Medicine, Shanghai, P.R.China) was added in each well and DNA incorporated radioactivity was measured afte an additional 16 to 18 hours by liquid scintillation counting. All proliferation assays were performed in triplicate.The data represent the mean counts per minute (cpm). Results were expressed as stimulation index (SI: after cpm incorporated in response to antigens minus cpm incorporated in the absence of antigens divided by cpm incorporated in the absence of antigens).SI2.1 is positive (or efficient) proliferative responses.
Determination of the cellular phenotype PBMC, which were stimulated with HCV antigens or not, were analyzed by immunofluorescence on a fluorescence activated cell sorter (FACS) after staining with the fluorescein isothiocyanate-conjugated monoclonal antibodies (anti-CD4,anti-CD8, Purchased from Dept. of Immunology Beijing Medical University, P.R.China) and specific fluorescence intensity was determined on a FACS ta flow cytometer (Becton & Dickinson).u|ax'/@, http://www.100md.com
Statistical analysis Results are expressed as mean ±S.Statistical analysis was performed using the student’s test. A level of P<0.05 was considered to be statistically significant.u|ax'/@, http://www.100md.com
RESULTSu|ax'/@, http://www.100md.com
Proliferation response of PBMC to different doses HCV synthetic peptides To select appropriate optimum antigens doses we first adopt 1μg, 4μg, 8μg and 12μg/ml HCV synthetic peptides and detected proliferation response of their PBMC to synthetic peptides CP9 and NS4. Fig 1 showed the proliferation response of their PBMC to different do-ses HCV synthetic peptides in vitro. PBMC from 8 individual with hepatitis C appear to be have diffe-rent proliferative responses to CP9 and NS4 in diffe-rent doses. As the synthetic peptides doses became higher the proliferative response of their PBMC to HCV synthetic peptides CP9 and NS4 were stronger gradually. However, proliferative response had no significant difference between 8μg dose group and 12μg dose group after the date was analyzed by student’s test. None of the HCV-RNA negative control individual responded to synthetic peptides CP9 and NS4.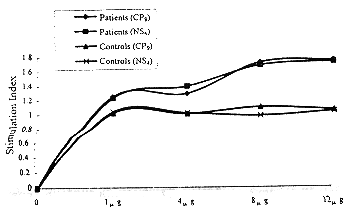
Fig 1 Dose-response curves of PBMC stimulated with HCV synthetic peptides CP9 and NS4nsa, http://www.100md.com
(PBMC (2×105 cells) from 8 individual with hepatitis C and 8 individual with HCV-seronegative were incubated for 6 days respectively with 1μg, 4μg, 8μg and 12μg/ml CP9 and NS4.Each point was a mean value of 8 samples.nsa, http://www.100md.com
Proliferative response of PBMC from patients with hepatits C to different HCV antigens The proliferative responses to HCV antigens in 24 patients with hepatitis C showed as table 1. PBMC from 25% (6/24) of these patients proliferated in response to at least one HCV antigens. According to the ratio of positive proliferative response(SI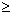 2.1) PBMC from patients with hepatitis C proliferative responses to recombinant HCV C,E1,E2 and NS3 proteins were 26.7%(4/15), 18.2%(2/11),18.2%(2/11)and 9.1%(1/11) respectively and proliferative responses to HCV synthetic peptides CP9, NS4 were 25% (6/24) and 16.7%(4/24) in order. The most frequent responses were to antigens of HCV C region.HCV-RNA column in Fig.1 showed that the two out of three patients with acute hepatitis C had efficient proliferative response of their PBMC to HCV antigens (SI
2.1) PBMC from patients with hepatitis C proliferative responses to recombinant HCV C,E1,E2 and NS3 proteins were 26.7%(4/15), 18.2%(2/11),18.2%(2/11)and 9.1%(1/11) respectively and proliferative responses to HCV synthetic peptides CP9, NS4 were 25% (6/24) and 16.7%(4/24) in order. The most frequent responses were to antigens of HCV C region.HCV-RNA column in Fig.1 showed that the two out of three patients with acute hepatitis C had efficient proliferative response of their PBMC to HCV antigens (SI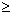 2.1) and their serum HCV-RNA converted negative and had normal level of ALT as well.
2.1) and their serum HCV-RNA converted negative and had normal level of ALT as well.
Tab 1 PBMC proliferative response to different HCV antigens in patients with hepatitis C Patient10jnqh, 百拇医药
no.10jnqh, 百拇医药
Age10jnqh, 百拇医药
(Yr)10jnqh, 百拇医药
Sex10jnqh, 百拇医药
Anti-10jnqh, 百拇医药
HCV10jnqh, 百拇医药
HCV-10jnqh, 百拇医药
RNA10jnqh, 百拇医药
Path10jnqh, 百拇医药
PBMC proliferation in response to10jnqh, 百拇医药
Synthetic peptides10jnqh, 百拇医药
Recombinent proteins10jnqh, 百拇医药
CP910jnqh, 百拇医药
NS410jnqh, 百拇医药
C10jnqh, 百拇医药
E110jnqh, 百拇医药
E210jnqh, 百拇医药
NS310jnqh, 百拇医药
110jnqh, 百拇医药
6510jnqh, 百拇医药
M10jnqh, 百拇医药
+10jnqh, 百拇医药
+10jnqh, 百拇医药
CHR10jnqh, 百拇医药
1.10
1.05!gi&fk!, 百拇医药
2!gi&fk!, 百拇医药
37!gi&fk!, 百拇医药
F!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
-!gi&fk!, 百拇医药
ACUΔ!gi&fk!, 百拇医药
2.27!gi&fk!, 百拇医药
2.13!gi&fk!, 百拇医药
3!gi&fk!, 百拇医药
28!gi&fk!, 百拇医药
M!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
CHR!gi&fk!, 百拇医药
1.46!gi&fk!, 百拇医药
1.55!gi&fk!, 百拇医药
4!gi&fk!, 百拇医药
48!gi&fk!, 百拇医药
M!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
CHR!gi&fk!, 百拇医药
1.81!gi&fk!, 百拇医药
1.85!gi&fk!, 百拇医药
5!gi&fk!, 百拇医药
54!gi&fk!, 百拇医药
F!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
CHR!gi&fk!, 百拇医药
2.79
1.13d}t., 百拇医药
6d}t., 百拇医药
28d}t., 百拇医药
Md}t., 百拇医药
+d}t., 百拇医药
+d}t., 百拇医药
CHRd}t., 百拇医药
1.80d}t., 百拇医药
2.03d}t., 百拇医药
7d}t., 百拇医药
61d}t., 百拇医药
Md}t., 百拇医药
+d}t., 百拇医药
-d}t., 百拇医药
CHRd}t., 百拇医药
1.08d}t., 百拇医药
2.14d}t., 百拇医药
8d}t., 百拇医药
29d}t., 百拇医药
Md}t., 百拇医药
+d}t., 百拇医药
+d}t., 百拇医药
CHRd}t., 百拇医药
1.96d}t., 百拇医药
1.77d}t., 百拇医药
9d}t., 百拇医药
27d}t., 百拇医药
Fd}t., 百拇医药
-d}t., 百拇医药
+d}t., 百拇医药
CHRd}t., 百拇医药
1.26
1.98o, http://www.100md.com
10o, http://www.100md.com
28o, http://www.100md.com
Mo, http://www.100md.com
+o, http://www.100md.com
+o, http://www.100md.com
CHRo, http://www.100md.com
1.91o, http://www.100md.com
1.74o, http://www.100md.com
2.68o, http://www.100md.com
2.68o, http://www.100md.com
11o, http://www.100md.com
34o, http://www.100md.com
Mo, http://www.100md.com
+o, http://www.100md.com
-o, http://www.100md.com
CHRo, http://www.100md.com
2.16o, http://www.100md.com
3.50o, http://www.100md.com
1.96o, http://www.100md.com
12o, http://www.100md.com
48o, http://www.100md.com
Mo, http://www.100md.com
+o, http://www.100md.com
+o, http://www.100md.com
CHRo, http://www.100md.com
1.80o, http://www.100md.com
1.47o, http://www.100md.com
1.48o, http://www.100md.com
13o, http://www.100md.com
27o, http://www.100md.com
F
+dqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
ACUΔdqj%!, http://www.100md.com
1.60dqj%!, http://www.100md.com
1.32dqj%!, http://www.100md.com
1.67dqj%!, http://www.100md.com
14dqj%!, http://www.100md.com
25dqj%!, http://www.100md.com
Mdqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
-dqj%!, http://www.100md.com
ACUΔdqj%!, http://www.100md.com
3.46dqj%!, http://www.100md.com
1.58dqj%!, http://www.100md.com
2.34dqj%!, http://www.100md.com
2.16dqj%!, http://www.100md.com
2.21dqj%!, http://www.100md.com
1.65dqj%!, http://www.100md.com
15dqj%!, http://www.100md.com
27dqj%!, http://www.100md.com
Fdqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
CHRdqj%!, http://www.100md.com
1.01dqj%!, http://www.100md.com
1.32dqj%!, http://www.100md.com
1.01dqj%!, http://www.100md.com
1.45dqj%!, http://www.100md.com
1.40dqj%!, http://www.100md.com
0.97dqj%!, http://www.100md.com
16
41+&{gx}*, 百拇医药
F+&{gx}*, 百拇医药
++&{gx}*, 百拇医药
++&{gx}*, 百拇医药
CHR+&{gx}*, 百拇医药
1.20+&{gx}*, 百拇医药
1.15+&{gx}*, 百拇医药
1.00+&{gx}*, 百拇医药
0.99+&{gx}*, 百拇医药
1.04+&{gx}*, 百拇医药
1.12+&{gx}*, 百拇医药
17+&{gx}*, 百拇医药
40+&{gx}*, 百拇医药
M+&{gx}*, 百拇医药
++&{gx}*, 百拇医药
++&{gx}*, 百拇医药
CHR+&{gx}*, 百拇医药
2.21+&{gx}*, 百拇医药
1.65+&{gx}*, 百拇医药
2.40+&{gx}*, 百拇医药
1.57+&{gx}*, 百拇医药
1.65+&{gx}*, 百拇医药
2.47+&{gx}*, 百拇医药
18+&{gx}*, 百拇医药
37+&{gx}*, 百拇医药
M+&{gx}*, 百拇医药
NT+&{gx}*, 百拇医药
++&{gx}*, 百拇医药
CHR+&{gx}*, 百拇医药
1.10+&{gx}*, 百拇医药
1.03+&{gx}*, 百拇医药
1.05/:, 百拇医药
1.32/:, 百拇医药
1.15/:, 百拇医药
1.02/:, 百拇医药
19/:, 百拇医药
58/:, 百拇医药
M/:, 百拇医药
+/:, 百拇医药
+/:, 百拇医药
CHR/:, 百拇医药
2.18/:, 百拇医药
2.10/:, 百拇医药
2.40/:, 百拇医药
1.62/:, 百拇医药
1.59/:, 百拇医药
1.50/:, 百拇医药
20/:, 百拇医药
49/:, 百拇医药
M/:, 百拇医药
NT/:, 百拇医药
+/:, 百拇医药
CHR/:, 百拇医药
1.28/:, 百拇医药
1.16/:, 百拇医药
1.09/:, 百拇医药
1.28/:, 百拇医药
1.10/:, 百拇医药
1.03/:, 百拇医药
21/:, 百拇医药
60/:, 百拇医药
M/:, 百拇医药
+7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
CHR7'w**^+, http://www.100md.com
1.247'w**^+, http://www.100md.com
1.387'w**^+, http://www.100md.com
2.087'w**^+, http://www.100md.com
2.347'w**^+, http://www.100md.com
2.237'w**^+, http://www.100md.com
1.177'w**^+, http://www.100md.com
227'w**^+, http://www.100md.com
327'w**^+, http://www.100md.com
M7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
CHR7'w**^+, http://www.100md.com
1.187'w**^+, http://www.100md.com
1.077'w**^+, http://www.100md.com
1.617'w**^+, http://www.100md.com
1.557'w**^+, http://www.100md.com
1.447'w**^+, http://www.100md.com
1.257'w**^+, http://www.100md.com
237'w**^+, http://www.100md.com
547'w**^+, http://www.100md.com
M7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
CHR7'w**^+, http://www.100md.com
1.457'w**^+, http://www.100md.com
1.227'w**^+, http://www.100md.com
1.347'w**^+, http://www.100md.com
1.037'w**^+, http://www.100md.com
1.055kk#, 百拇医药
1.235kk#, 百拇医药
245kk#, 百拇医药
305kk#, 百拇医药
M5kk#, 百拇医药
+5kk#, 百拇医药
+5kk#, 百拇医药
CHR5kk#, 百拇医药
1.185kk#, 百拇医药
1.045kk#, 百拇医药
1.025kk#, 百拇医药
1.035kk#, 百拇医药
1.245kk#, 百拇医药
1.17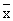 ±s5kk#, 百拇医药
±s5kk#, 百拇医药
1.695kk#, 百拇医药
1.615kk#, 百拇医药
1.685kk#, 百拇医药
1.495kk#, 百拇医药
1.445kk#, 百拇医药
1.335kk#, 百拇医药
±0.635kk#, 百拇医药
±0.555kk#, 百拇医药
±0.585kk#, 百拇医药
±0.445kk#, 百拇医药
0.44±5kk#, 百拇医药
±0.435kk#, 百拇医药
Ratio of positive response5kk#, 百拇医药
6/245kk#, 百拇医药
4/24
4/154me'y, 百拇医药
2/114me'y, 百拇医药
2/114me'y, 百拇医药
1/114me'y, 百拇医药
Δ ACU, acute hepatitis C;CHR, chronic hepatitis C;A,abnormal; N, normal; NT, no test;4me'y, 百拇医药
* Serum ALT values measured on the same day as when PBMC proliferation was assessed.4me'y, 百拇医药
—, Positive proliferation responses was SI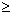 2.1.4me'y, 百拇医药
2.1.4me'y, 百拇医药
Phenotype analysis To determine which type of cells proliferated in response to HCV antigens in vitro phenotypic characteristics of proliferating cells in PBMC cultivation for 6 days with HCV antigens were detected by immunofluorescence on FACS. Table 2 showed that the PBMC from patients with chronic C appeared to be proliferated after stimulated with HCV antigens and phenotypic characteristics of proliferating cells was CD4+ lymphocyte and phenotypic characteristics of CD8+ lymphocyte was less than that of CD+4 lymphocyte after stimulated with HCV antigons, of which CD+4
Tab 2 Phenotypic characteristics of proliferating cells sti-$':v, 百拇医药
mulated with HCV antigens Cells$':v, 百拇医药
Phenotype$':v, 百拇医药
no stimulated$':v, 百拇医药
with HCV antigens$':v, 百拇医药
Stimulated with HCV antigens$':v, 百拇医药
CP9$':v, 百拇医药
NS4$':v, 百拇医药
C$':v, 百拇医药
CD+4$':v, 百拇医药
40.05±3.48$':v, 百拇医药
44.34±1.69Δ$':v, 百拇医药
43.48±1.92$':v, 百拇医药
44.28±4.46Δ$':v, 百拇医药
CD+8$':v, 百拇医药
20.27±1.27$':v, 百拇医药
21.19±2.34$':v, 百拇医药
21.56±2.33$':v, 百拇医药
22.5±2.59$':v, 百拇医药
CD+4/CD+8$':v, 百拇医药
1.97±0.19$':v, 百拇医药
2.09±0.24
2.02±0.18+}, http://www.100md.com
1.99±0.21+}, http://www.100md.com
*each value was a mean of 5 samples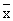 ±s+}, http://www.100md.com
±s+}, http://www.100md.com
Δcompared with no HCV antigens stimulating,P<0.05+}, http://www.100md.com
PBMC were cultured for 6 days in the presence of HCV synthetic peptides CP9, NS4 and HCV recombinant C protein or not.+}, http://www.100md.com
lymphocyte proliferative responses to HCV synthetic peptide CP9 and recombinant antigen C had significant increased in comparison with HCV antigens stimulating before.+}, http://www.100md.com
DISCUSSION+}, http://www.100md.com
Persistence of HCV in the infected host is a very common event that occurs in more than 60% of infected patients. The complex interplay between virus and immune cells and the possible viral interference with an efficient development of the protective immure response are still to be elucida-ted[6]. In particular, information on the role played by T and B cells with selective specificity for different HCV antigens in the neutralization of circulating and intracellular virus is still poorly understood.
To gain insight into the immune function responsible for clearing viral infection we detected the lymphoproliferative response to HCV antigens in 24 patients with hepatitis C. In preliminary experiment 8 patients with chronic hepatitis C and 8 healthy individuals were studied for PBMC proliferative response to different concentrations (1μg, 4μg, 8μg and 12 μg/ml) of HCV synthetic peptide CP9 and NS4. The result showed that lymphoproliferative response was more vigorous in presence of higher dose peptide antigens and that proliferative response of their PBMC was stronger in 12μg/ml dose group than that of 8 mg/ml dose group. However, there was no statistical significance between the two dose groups. In order to show the nature of proliferation response of PBMC from patients with hepatitis C and save expensive HCV antigens 8μg/ml antigens dose was taken in later experiment.*, http://www.100md.com
The study shows that the majority of HCV-infected patients exhibit CD+4 T-cell responses to viral antigens. This was established by assessing the HCV-specific PBMC proliferative response in vitro, which depeneds on the presence of T cells that have been exposed to HCV antigens and have therefore undergone clonal expansion in vivo. However, the proliferative response intensity of PBMC is less than that of other’s report[3~6]. These are possible correlated with lower titer of HCV in infected body, inferior HCV immunogen, weak immune responses to HCV antigens in infec-ted host and not much purity of HCV antigens used in our experiment. From the point of view of selecting HCV predominant antigens core antigens (recombinant HCV C protein and synthetic peptide CP9) were a good immunogen because the PBMC proliferative responses to core antigens were the most vigorous in comparison with PBMC proliferative responses stimulated with any other HCV antigens (recombinant HCV E1,E2, NS3 and synthetic peptide NS4). This investigation result can provide a feasible method from cellular immune prevention for selecting HCV predominant antigens used in development HCV vaccine in future.
The phenotypic characteristics of proliferating cells indicated that HCV-specific CD+4 T lymphocytes proliferated more vigorous than that of CD+8 T lymphocytes measured by FACS. The result can suggest the PBMC stimulated with soluble exogenous antigens is expected to induce a preferential activation of CD4-positive HLA class Ⅱ-restricted T cells and not of CD8-positive HLA class Ⅰ-res-tricted cytotoxic T lymphocytes which are believed to be the main effectors for the elimination of virus-infected cells[11,12]. Because exogenous antigens are phagocytized and dealed with by macrophange and present antigens to the HLA-calss Ⅱ molecules to form heteromolecular complexes that are recognized by CD+4 T lymphocytes and induce CD+4 T lymphocytes to expand and proliferate.w, 百拇医药
The relationship between the immune res-ponse of PBMC from patients with hepatitis C to HCV antigens and outcome of infection is now difficult to determine. There are a few different opinions in some researchers([3~7]. Our study showed that the efficient proliferation response (SI2.1) of PBMC from patients with acute hepatitis C likely indicated that the lymphoproliferative response to HCV antigens are associated with clinical prognosis of HCV infection. Indeed, in the patients with acute hepatitis C, whose PBMC proliferative response to HCV antigens are stronger, their serum HCV-RNA were negative and serum ALT were normal when studied. In the other patients with acute and chronic hepatitis C, whose PBMC did not demonstrate efficient proliferative responses to HCV antigens, their serum HCV-RNA were detectable and serum ALT were elevated. Because the cases studied is not enough further studies in a large group of patients with hepatitis C and using more various antigens in PBMC proliferation assay are required. The present findings may show that PBMC from patients with hepatitis C exist proliferative responses to HCV proteins/peptides, of which core antigens were a good immunogen than any other HCV antigens used in our studies and suggest that the significant lympho-proliferative responses to HCV antigens are more frequently observed in acute infective patients who were able to eradicate the virus than in chronic HCV infective patients.
*This study was supported by the science foundation of public healih bureau of Zhejiang province in China.$5+]61f, 百拇医药
作者单位:Dou Jun(窦骏) Liu Kezhou(刘克洲) Chen Zhi(陈智) Wo Jian’er(沃建尔)He Nanxiang(何南祥) Xu Chenhuai(徐陈槐) Zhang Mingtai(章明太) Wang Xinzi(王信子) Institue of Infectius Disease ,Zhejiang Medical University ,Hangzhou 310003(Dou Jun now at:Department of Microbiology Nanjing Railway Medical College,Nanjing 210009)$5+]61f, 百拇医药
第一作者:男,43岁,博士,副教授$5+]61f, 百拇医药
REFERENCES$5+]61f, 百拇医药
[1] Dienstang JL. Hepatitis non-A, non-B:C at last. Gastroenterology, 1990,99:1177$5+]61f, 百拇医药
[2] Aler MJ,Margolis HS, Krawczynski K, et al. The natural history of community-acquired hepatitis C in the United statas. N Engl J Med, 1992,327:1899$5+]61f, 百拇医药
[3] Lechmann M, Ihlenfeldt HG, Braunschweiger I, et al. T-and B-cell responses to different hepatitis C virus antigens in patients with chronic hepatitis C infection and in healthy anti-hepatitis C virus-positive blood donors without viremia. Hepatology, 1996,24:790$5+]61f, 百拇医药
[4] Schupper H,Hayashi P,Scheffel J, et al. Peripheral-blood mononuclear cell responses to recombinant hepa-titis C virus antigens in patients with chronic hepatitis C. Hepatology, 1993,18:1055
[5] Botarelli P,Brunetto MR, Minutello MA, et al. T-lymphocyte response to hepatitis C virus in different clinical courses of infection.Gastroenterology, 1993,104:5082, http://www.100md.com
[6] Ferrari C,Valli A, Galati L, et al. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology, 1994,19:2862, http://www.100md.com
[7] Geert LR, Esquivel CA,Deleys R, et al. Lymphoproliferative responses to hepatitis C virus Core, E1,E2,and E3 in patients with chronic hepatitis C infection treated with interferon alfa. Hepatology, 1996,23:82, http://www.100md.com
[8] Kozied MJ,Dudley D,Afdhal N, et al. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C in persons with chronic hepatitis. J Immunol, 1992,149:33392, http://www.100md.com
[9] Han JH, shyanala V, Richman KH, et al. Characterization of terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5’ untranslated region and poly (A) tails at the 3’ end. Proc Natl Acad Sci USA,1991,88:17112, http://www.100md.com
[10] Chomczynski P, Sacchi N.Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem, 1987,162:1562, http://www.100md.com
[11] Kita H,Moriyama T, Kaneko T, et al. HLAB44-res-tricted cytotoxic T lymphocytes recognizing an epitope on hepatitis C virus nucleocasid protein. Hepatology, 1993, 18:10392, http://www.100md.com
[12] Kita H,Moriyama T,Kaneko T, et al. A Helper T-cell antigen enhances generation of hepatitis C virus-specific cytotoxic T lymphocytes in Vitro. T Med Virol, 1995, 45:3862, http://www.100md.com
(1998-04-03收稿;1998-07-13修回)(Dou Jun(窦骏), Liu Kezhou(刘克洲),Chen Zhi(陈智)1, Wo Jian’er(沃建尔),He Nanxiang(何南祥))
单位:]n, http://www.100md.com
关键词:丙型肝炎病毒 丙型肝炎病人 外周血单个核细胞 细胞免疫]n, http://www.100md.com
免疫学杂志990102 摘 要 为探讨丙型肝炎(HC)病人细胞免疫功能和丙型肝炎病毒(HCV)的致病机及机体对其免疫保护作用,收集24例HC病人(急性3例,慢性21例),用3H-TdR掺入法研究病人外周血单个核细胞(PBMC)对不同HCV抗原增殖反应,并用流式细胞仪(FACS)检测了PBMC中CD4+、CD8+淋巴细胞亚群在HCV抗原刺激后的变化。结果:HC病人PBMC对HCV合成肽CP9,NS4和基因重组抗原C,E1,E2,NS3刺激后出现不同程度增殖反应,刺激指数(SI)分别为1.69±0.51,1.61±0.54,1.68±0.58,1.49±0.44,1.44±0.44和1.33±0.33。3例急性HC中2例病人的PBMC对HCV抗原呈有效增殖反应(SI≥2.1),且血清HCVRNA阴转伴ALT正常。细胞表型分析显示:增殖的细胞表型是CD4+淋巴细胞,而CD8+淋巴细胞增殖反应较弱。结论:HC病人PBMC确实存在对HCV抗原的增殖反应;CD4+淋巴细胞比CD8+淋巴细胞增殖反应要强,急性HC病人PBMC对HCV抗原有效的增殖反应预示可能有良好的临床愈合。]n, http://www.100md.com
中图号 R374.21]n, http://www.100md.com
Abstract To explore the cellular immune function in patients with hepatitis C and study the HCV pathogenic mechanism and immune protection effect against HCV infection in host. PBMC proliferative responses in vitro to HCV synthetic peptides (Cp9, Ns4) and recombinant HCV proteins (C,E1,E2,NS3) were evaluated in 21 patients with chronic hepatitis C and 3 patients with acute hepatitis C. Phenotypic characteristics of proliferating cells in PBMC were detected by FACS after PBMC were stimulated with HCV antigens. Results showed that lymphoproliferative responses of PBMC from patients with hepatitis C to synthetic peptides (Cp9, NS4) and recombinant proteins (C,E1,E2 and NS3) were detected and its stimulation index(SI) were 1.69±0.51, 1.61±0.54, 1.68±0.58, 1.49±0.44, 1.44±0.44 and 1.33±0.33 respectively. Two out of three patients with acute hepatitis C had efficient lymphoproliferative response to HCV antigens (SI
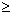 2.1) and their serum HCV-RNA were seroconversion and serum ALT values were returned to normal when studied. Phenotype analysis showed that phenotypic characteristics of proliferating cells was CD4+ lymphocytes and CD8+ lymphocytes proliferative responses was less than that of CD4+ lymphocytes. The results indicate that PBMC from patients with hepatitis C exist proliferative responses to different HCV antigens. CD4+ lymphocytes proliferative response is greater than that of CD8+ lymphocytes. The efficient lymphoproliferative responses to HCV antigens in patients with acute hepatitis C suggests that the patients possibly have a good clinical prognosis.
2.1) and their serum HCV-RNA were seroconversion and serum ALT values were returned to normal when studied. Phenotype analysis showed that phenotypic characteristics of proliferating cells was CD4+ lymphocytes and CD8+ lymphocytes proliferative responses was less than that of CD4+ lymphocytes. The results indicate that PBMC from patients with hepatitis C exist proliferative responses to different HCV antigens. CD4+ lymphocytes proliferative response is greater than that of CD8+ lymphocytes. The efficient lymphoproliferative responses to HCV antigens in patients with acute hepatitis C suggests that the patients possibly have a good clinical prognosis.Key words Hepatitis C virus,Patients with hepatitis C,Peripheral blood mononuclear cell,Cellular immunet8, http://www.100md.com
Hepatitis C virus (HCV) has been identified as a major etiological agent of parentally and community-acquired NANB hepatitis and most patients with hepatitis C are not able to eliminate the virus[1~2]. However, it is still not known that HCV pathogenic mechanism and immune protecting effect against HCV infection in host at present. The presence of antibodies to most HCV antigens in patients with chronic hepatitis C suggests that the humoral immune responses to the virus is unable to stop the progression of disease[3~8]. In order to explore the cellular immune function in patients with hepatitis C peripheral blood mononuclear cell (PBMC) proliferative res-ponses in vitro to HCV synthetic peptides (Cp9,NS4) and gene recombinant HCV proteins (C,E1,E2,NS3) were evaluated in 21 patients with chronic hepatitis C and 3 patients with acute hepatitis C. We are try to analysis whether there are a relationship between PBMC immune responses in patients with hepatitis C to various HCV antigens and clinical prognosis of HCV infection and to select HCV predominant antigens which are to be thought of having a good immunogenicity and provide the basis for studing the function of PBMC from patients with hepatitis C and HCV vaccine development in future.
MATERIAL AND METHODS9z\6), http://www.100md.com
HCV-seropositive individuals For the present study we selected 24 individuals with serum HCV-RNA positive detected by reverse transcription-nested polymerase chain reaction (RT-nested-PCR). The assay was performed as previously described[9], Briefly, RNA was extracted from 50μl of serum by a modification of a method described elsewhere[10]. After reverse transcription at 42°C for 90 minutes, complementary DNA was amplified by two round PCR by using primers from the core region of HCV genome. (positive sense:5′ G G T C G C A A C C T C G T G G A A G G 3′,negative sense:5′A T C G A T G A C C T T A C C C A A A T 3′). The amplification profile was as follows:denaturation at 95°C for 1 minute, annealing at 55°C for 1 minute and extension at 72°C for 45 seconds, total of 30 cycles. PCR products (209pb) were subjected to electrophoresis in 2% agarose gels, stained with ethidium bremide, and visualized under ultraviolet illumination.9z\6), http://www.100md.com
Patients with chronic hepatitis C This group consisted of 21 patients [17 male and 4 female; median age: 44 years, (range, 27~65)]who had elevated serum levels(>40IU/L)of alanine amino transferase(ALT) for 1 year and liver histology compatible with the diagnosis of chronic hepatitis by the diagnosis criterion of viral hepatitis prevention and treatment in the fifth national symposium on infectious diseases and parastie diseases, May, 1995, Beijing, P.R.China.
Patients with acute hepatitis C This group consisted of 3 individuals [1 male and 2 female,median age: 30 years (range, 25~37)] with a history of blood transfusion, whose serum levels of ALT>40 IU/L for no more than 3 months and whose signs and symptoms were compatible with the dia-gnosis of acute hepatitis by the diagnosis criterion of viral hepatitis prevention and treatment in the fifth national symposium on infectious diseases and parasite diseases, May, 1995, Beijing, P.R.China.o96]li*, http://www.100md.com
HCV-seronegative individuals As controls for the specificity of PBMC responses,we selected 8 healthy individuals without a clinical history of hepatitis and without symptoms or signs of liver disease and serum HCV RNA negative [6 male and 2 female, median age: 29 years (range, 21~41)].o96]li*, http://www.100md.com
HCV antigens HCV recombinant proteins: The HCV proteins core, envelope 1(E1), envelope 2 (E2) nonstructural 3(NS3) were supplied and gifted by prof. Zhuang Hui (Dept. of Microbiology, Beijing Medical University, P.R.China) and Dr.wang Xingtai, Dr. Zhang Shuming (Dept. of hepatitis, national institute for the control of pharmaceutical and biological products. Beijing, P.R.China).
HCV synthetic peptides: The HCV synthetic peptides Cp9,NS4 were syntheted by Institute of Biochemistry Chinese Academy of Medicine, Shanghai, P.R.China).$?{e#%, 百拇医药
Cell Culture-medium The medium used of all cell cultures consisted of RPMI 1640 supplemented with 25 mmol/L HEPES, 2 mmol/L L-glutamine (all from GibcoBRL,Scotland), Penicillin (100u/ml), streptomycin (100μg/ml) and 5% (voL/voL) pooled human serum(RPMI-HS). The AB serum was obtained from healthy blood donors with blood group AB+ and was only used when antibodies to HCV were absent.$?{e#%, 百拇医药
PBMC proliferation assay Heparinized venous blood drawn from patients with hepatitis C were diluted in Hank’s liquid. PBMC were separated by Ficoll-Hypaque (The second agent factory, Shanghai, P.R.China)density gradient centrifugation. Proliferation assays were performed as described [4-5]. Briefly, PBMC(2×105 cells) in 0.2 ml of RPMI-HS were cultured in 96-well flat-bottom microplates in the presence or absence of HCV synthetic peptides or HCV recombinant proteins at various final concentration for 6 days at 37°C in an atmosphere of 5% CO2 in air. 0.5 μCi of [3H] thymidine (3H-TdR,Purchased from Institute of radionuelide, Chinese Academy of Medicine, Shanghai, P.R.China) was added in each well and DNA incorporated radioactivity was measured afte an additional 16 to 18 hours by liquid scintillation counting. All proliferation assays were performed in triplicate.The data represent the mean counts per minute (cpm). Results were expressed as stimulation index (SI: after cpm incorporated in response to antigens minus cpm incorporated in the absence of antigens divided by cpm incorporated in the absence of antigens).SI2.1 is positive (or efficient) proliferative responses.
Determination of the cellular phenotype PBMC, which were stimulated with HCV antigens or not, were analyzed by immunofluorescence on a fluorescence activated cell sorter (FACS) after staining with the fluorescein isothiocyanate-conjugated monoclonal antibodies (anti-CD4,anti-CD8, Purchased from Dept. of Immunology Beijing Medical University, P.R.China) and specific fluorescence intensity was determined on a FACS ta flow cytometer (Becton & Dickinson).u|ax'/@, http://www.100md.com
Statistical analysis Results are expressed as mean ±S.Statistical analysis was performed using the student’s test. A level of P<0.05 was considered to be statistically significant.u|ax'/@, http://www.100md.com
RESULTSu|ax'/@, http://www.100md.com
Proliferation response of PBMC to different doses HCV synthetic peptides To select appropriate optimum antigens doses we first adopt 1μg, 4μg, 8μg and 12μg/ml HCV synthetic peptides and detected proliferation response of their PBMC to synthetic peptides CP9 and NS4. Fig 1 showed the proliferation response of their PBMC to different do-ses HCV synthetic peptides in vitro. PBMC from 8 individual with hepatitis C appear to be have diffe-rent proliferative responses to CP9 and NS4 in diffe-rent doses. As the synthetic peptides doses became higher the proliferative response of their PBMC to HCV synthetic peptides CP9 and NS4 were stronger gradually. However, proliferative response had no significant difference between 8μg dose group and 12μg dose group after the date was analyzed by student’s test. None of the HCV-RNA negative control individual responded to synthetic peptides CP9 and NS4.

Fig 1 Dose-response curves of PBMC stimulated with HCV synthetic peptides CP9 and NS4nsa, http://www.100md.com
(PBMC (2×105 cells) from 8 individual with hepatitis C and 8 individual with HCV-seronegative were incubated for 6 days respectively with 1μg, 4μg, 8μg and 12μg/ml CP9 and NS4.Each point was a mean value of 8 samples.nsa, http://www.100md.com
Proliferative response of PBMC from patients with hepatits C to different HCV antigens The proliferative responses to HCV antigens in 24 patients with hepatitis C showed as table 1. PBMC from 25% (6/24) of these patients proliferated in response to at least one HCV antigens. According to the ratio of positive proliferative response(SI
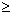 2.1) PBMC from patients with hepatitis C proliferative responses to recombinant HCV C,E1,E2 and NS3 proteins were 26.7%(4/15), 18.2%(2/11),18.2%(2/11)and 9.1%(1/11) respectively and proliferative responses to HCV synthetic peptides CP9, NS4 were 25% (6/24) and 16.7%(4/24) in order. The most frequent responses were to antigens of HCV C region.HCV-RNA column in Fig.1 showed that the two out of three patients with acute hepatitis C had efficient proliferative response of their PBMC to HCV antigens (SI
2.1) PBMC from patients with hepatitis C proliferative responses to recombinant HCV C,E1,E2 and NS3 proteins were 26.7%(4/15), 18.2%(2/11),18.2%(2/11)and 9.1%(1/11) respectively and proliferative responses to HCV synthetic peptides CP9, NS4 were 25% (6/24) and 16.7%(4/24) in order. The most frequent responses were to antigens of HCV C region.HCV-RNA column in Fig.1 showed that the two out of three patients with acute hepatitis C had efficient proliferative response of their PBMC to HCV antigens (SI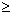 2.1) and their serum HCV-RNA converted negative and had normal level of ALT as well.
2.1) and their serum HCV-RNA converted negative and had normal level of ALT as well.Tab 1 PBMC proliferative response to different HCV antigens in patients with hepatitis C Patient10jnqh, 百拇医药
no.10jnqh, 百拇医药
Age10jnqh, 百拇医药
(Yr)10jnqh, 百拇医药
Sex10jnqh, 百拇医药
Anti-10jnqh, 百拇医药
HCV10jnqh, 百拇医药
HCV-10jnqh, 百拇医药
RNA10jnqh, 百拇医药
Path10jnqh, 百拇医药
PBMC proliferation in response to10jnqh, 百拇医药
Synthetic peptides10jnqh, 百拇医药
Recombinent proteins10jnqh, 百拇医药
CP910jnqh, 百拇医药
NS410jnqh, 百拇医药
C10jnqh, 百拇医药
E110jnqh, 百拇医药
E210jnqh, 百拇医药
NS310jnqh, 百拇医药
110jnqh, 百拇医药
6510jnqh, 百拇医药
M10jnqh, 百拇医药
+10jnqh, 百拇医药
+10jnqh, 百拇医药
CHR10jnqh, 百拇医药
1.10
1.05!gi&fk!, 百拇医药
2!gi&fk!, 百拇医药
37!gi&fk!, 百拇医药
F!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
-!gi&fk!, 百拇医药
ACUΔ!gi&fk!, 百拇医药
2.27!gi&fk!, 百拇医药
2.13!gi&fk!, 百拇医药
3!gi&fk!, 百拇医药
28!gi&fk!, 百拇医药
M!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
CHR!gi&fk!, 百拇医药
1.46!gi&fk!, 百拇医药
1.55!gi&fk!, 百拇医药
4!gi&fk!, 百拇医药
48!gi&fk!, 百拇医药
M!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
CHR!gi&fk!, 百拇医药
1.81!gi&fk!, 百拇医药
1.85!gi&fk!, 百拇医药
5!gi&fk!, 百拇医药
54!gi&fk!, 百拇医药
F!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
+!gi&fk!, 百拇医药
CHR!gi&fk!, 百拇医药
2.79
1.13d}t., 百拇医药
6d}t., 百拇医药
28d}t., 百拇医药
Md}t., 百拇医药
+d}t., 百拇医药
+d}t., 百拇医药
CHRd}t., 百拇医药
1.80d}t., 百拇医药
2.03d}t., 百拇医药
7d}t., 百拇医药
61d}t., 百拇医药
Md}t., 百拇医药
+d}t., 百拇医药
-d}t., 百拇医药
CHRd}t., 百拇医药
1.08d}t., 百拇医药
2.14d}t., 百拇医药
8d}t., 百拇医药
29d}t., 百拇医药
Md}t., 百拇医药
+d}t., 百拇医药
+d}t., 百拇医药
CHRd}t., 百拇医药
1.96d}t., 百拇医药
1.77d}t., 百拇医药
9d}t., 百拇医药
27d}t., 百拇医药
Fd}t., 百拇医药
-d}t., 百拇医药
+d}t., 百拇医药
CHRd}t., 百拇医药
1.26
1.98o, http://www.100md.com
10o, http://www.100md.com
28o, http://www.100md.com
Mo, http://www.100md.com
+o, http://www.100md.com
+o, http://www.100md.com
CHRo, http://www.100md.com
1.91o, http://www.100md.com
1.74o, http://www.100md.com
2.68o, http://www.100md.com
2.68o, http://www.100md.com
11o, http://www.100md.com
34o, http://www.100md.com
Mo, http://www.100md.com
+o, http://www.100md.com
-o, http://www.100md.com
CHRo, http://www.100md.com
2.16o, http://www.100md.com
3.50o, http://www.100md.com
1.96o, http://www.100md.com
12o, http://www.100md.com
48o, http://www.100md.com
Mo, http://www.100md.com
+o, http://www.100md.com
+o, http://www.100md.com
CHRo, http://www.100md.com
1.80o, http://www.100md.com
1.47o, http://www.100md.com
1.48o, http://www.100md.com
13o, http://www.100md.com
27o, http://www.100md.com
F
+dqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
ACUΔdqj%!, http://www.100md.com
1.60dqj%!, http://www.100md.com
1.32dqj%!, http://www.100md.com
1.67dqj%!, http://www.100md.com
14dqj%!, http://www.100md.com
25dqj%!, http://www.100md.com
Mdqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
-dqj%!, http://www.100md.com
ACUΔdqj%!, http://www.100md.com
3.46dqj%!, http://www.100md.com
1.58dqj%!, http://www.100md.com
2.34dqj%!, http://www.100md.com
2.16dqj%!, http://www.100md.com
2.21dqj%!, http://www.100md.com
1.65dqj%!, http://www.100md.com
15dqj%!, http://www.100md.com
27dqj%!, http://www.100md.com
Fdqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
+dqj%!, http://www.100md.com
CHRdqj%!, http://www.100md.com
1.01dqj%!, http://www.100md.com
1.32dqj%!, http://www.100md.com
1.01dqj%!, http://www.100md.com
1.45dqj%!, http://www.100md.com
1.40dqj%!, http://www.100md.com
0.97dqj%!, http://www.100md.com
16
41+&{gx}*, 百拇医药
F+&{gx}*, 百拇医药
++&{gx}*, 百拇医药
++&{gx}*, 百拇医药
CHR+&{gx}*, 百拇医药
1.20+&{gx}*, 百拇医药
1.15+&{gx}*, 百拇医药
1.00+&{gx}*, 百拇医药
0.99+&{gx}*, 百拇医药
1.04+&{gx}*, 百拇医药
1.12+&{gx}*, 百拇医药
17+&{gx}*, 百拇医药
40+&{gx}*, 百拇医药
M+&{gx}*, 百拇医药
++&{gx}*, 百拇医药
++&{gx}*, 百拇医药
CHR+&{gx}*, 百拇医药
2.21+&{gx}*, 百拇医药
1.65+&{gx}*, 百拇医药
2.40+&{gx}*, 百拇医药
1.57+&{gx}*, 百拇医药
1.65+&{gx}*, 百拇医药
2.47+&{gx}*, 百拇医药
18+&{gx}*, 百拇医药
37+&{gx}*, 百拇医药
M+&{gx}*, 百拇医药
NT+&{gx}*, 百拇医药
++&{gx}*, 百拇医药
CHR+&{gx}*, 百拇医药
1.10+&{gx}*, 百拇医药
1.03+&{gx}*, 百拇医药
1.05/:, 百拇医药
1.32/:, 百拇医药
1.15/:, 百拇医药
1.02/:, 百拇医药
19/:, 百拇医药
58/:, 百拇医药
M/:, 百拇医药
+/:, 百拇医药
+/:, 百拇医药
CHR/:, 百拇医药
2.18/:, 百拇医药
2.10/:, 百拇医药
2.40/:, 百拇医药
1.62/:, 百拇医药
1.59/:, 百拇医药
1.50/:, 百拇医药
20/:, 百拇医药
49/:, 百拇医药
M/:, 百拇医药
NT/:, 百拇医药
+/:, 百拇医药
CHR/:, 百拇医药
1.28/:, 百拇医药
1.16/:, 百拇医药
1.09/:, 百拇医药
1.28/:, 百拇医药
1.10/:, 百拇医药
1.03/:, 百拇医药
21/:, 百拇医药
60/:, 百拇医药
M/:, 百拇医药
+7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
CHR7'w**^+, http://www.100md.com
1.247'w**^+, http://www.100md.com
1.387'w**^+, http://www.100md.com
2.087'w**^+, http://www.100md.com
2.347'w**^+, http://www.100md.com
2.237'w**^+, http://www.100md.com
1.177'w**^+, http://www.100md.com
227'w**^+, http://www.100md.com
327'w**^+, http://www.100md.com
M7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
CHR7'w**^+, http://www.100md.com
1.187'w**^+, http://www.100md.com
1.077'w**^+, http://www.100md.com
1.617'w**^+, http://www.100md.com
1.557'w**^+, http://www.100md.com
1.447'w**^+, http://www.100md.com
1.257'w**^+, http://www.100md.com
237'w**^+, http://www.100md.com
547'w**^+, http://www.100md.com
M7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
+7'w**^+, http://www.100md.com
CHR7'w**^+, http://www.100md.com
1.457'w**^+, http://www.100md.com
1.227'w**^+, http://www.100md.com
1.347'w**^+, http://www.100md.com
1.037'w**^+, http://www.100md.com
1.055kk#, 百拇医药
1.235kk#, 百拇医药
245kk#, 百拇医药
305kk#, 百拇医药
M5kk#, 百拇医药
+5kk#, 百拇医药
+5kk#, 百拇医药
CHR5kk#, 百拇医药
1.185kk#, 百拇医药
1.045kk#, 百拇医药
1.025kk#, 百拇医药
1.035kk#, 百拇医药
1.245kk#, 百拇医药
1.17
 ±s5kk#, 百拇医药
±s5kk#, 百拇医药1.695kk#, 百拇医药
1.615kk#, 百拇医药
1.685kk#, 百拇医药
1.495kk#, 百拇医药
1.445kk#, 百拇医药
1.335kk#, 百拇医药
±0.635kk#, 百拇医药
±0.555kk#, 百拇医药
±0.585kk#, 百拇医药
±0.445kk#, 百拇医药
0.44±5kk#, 百拇医药
±0.435kk#, 百拇医药
Ratio of positive response5kk#, 百拇医药
6/245kk#, 百拇医药
4/24
4/154me'y, 百拇医药
2/114me'y, 百拇医药
2/114me'y, 百拇医药
1/114me'y, 百拇医药
Δ ACU, acute hepatitis C;CHR, chronic hepatitis C;A,abnormal; N, normal; NT, no test;4me'y, 百拇医药
* Serum ALT values measured on the same day as when PBMC proliferation was assessed.4me'y, 百拇医药
—, Positive proliferation responses was SI
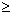 2.1.4me'y, 百拇医药
2.1.4me'y, 百拇医药Phenotype analysis To determine which type of cells proliferated in response to HCV antigens in vitro phenotypic characteristics of proliferating cells in PBMC cultivation for 6 days with HCV antigens were detected by immunofluorescence on FACS. Table 2 showed that the PBMC from patients with chronic C appeared to be proliferated after stimulated with HCV antigens and phenotypic characteristics of proliferating cells was CD4+ lymphocyte and phenotypic characteristics of CD8+ lymphocyte was less than that of CD+4 lymphocyte after stimulated with HCV antigons, of which CD+4
Tab 2 Phenotypic characteristics of proliferating cells sti-$':v, 百拇医药
mulated with HCV antigens Cells$':v, 百拇医药
Phenotype$':v, 百拇医药
no stimulated$':v, 百拇医药
with HCV antigens$':v, 百拇医药
Stimulated with HCV antigens$':v, 百拇医药
CP9$':v, 百拇医药
NS4$':v, 百拇医药
C$':v, 百拇医药
CD+4$':v, 百拇医药
40.05±3.48$':v, 百拇医药
44.34±1.69Δ$':v, 百拇医药
43.48±1.92$':v, 百拇医药
44.28±4.46Δ$':v, 百拇医药
CD+8$':v, 百拇医药
20.27±1.27$':v, 百拇医药
21.19±2.34$':v, 百拇医药
21.56±2.33$':v, 百拇医药
22.5±2.59$':v, 百拇医药
CD+4/CD+8$':v, 百拇医药
1.97±0.19$':v, 百拇医药
2.09±0.24
2.02±0.18+}, http://www.100md.com
1.99±0.21+}, http://www.100md.com
*each value was a mean of 5 samples
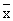 ±s+}, http://www.100md.com
±s+}, http://www.100md.comΔcompared with no HCV antigens stimulating,P<0.05+}, http://www.100md.com
PBMC were cultured for 6 days in the presence of HCV synthetic peptides CP9, NS4 and HCV recombinant C protein or not.+}, http://www.100md.com
lymphocyte proliferative responses to HCV synthetic peptide CP9 and recombinant antigen C had significant increased in comparison with HCV antigens stimulating before.+}, http://www.100md.com
DISCUSSION+}, http://www.100md.com
Persistence of HCV in the infected host is a very common event that occurs in more than 60% of infected patients. The complex interplay between virus and immune cells and the possible viral interference with an efficient development of the protective immure response are still to be elucida-ted[6]. In particular, information on the role played by T and B cells with selective specificity for different HCV antigens in the neutralization of circulating and intracellular virus is still poorly understood.
To gain insight into the immune function responsible for clearing viral infection we detected the lymphoproliferative response to HCV antigens in 24 patients with hepatitis C. In preliminary experiment 8 patients with chronic hepatitis C and 8 healthy individuals were studied for PBMC proliferative response to different concentrations (1μg, 4μg, 8μg and 12 μg/ml) of HCV synthetic peptide CP9 and NS4. The result showed that lymphoproliferative response was more vigorous in presence of higher dose peptide antigens and that proliferative response of their PBMC was stronger in 12μg/ml dose group than that of 8 mg/ml dose group. However, there was no statistical significance between the two dose groups. In order to show the nature of proliferation response of PBMC from patients with hepatitis C and save expensive HCV antigens 8μg/ml antigens dose was taken in later experiment.*, http://www.100md.com
The study shows that the majority of HCV-infected patients exhibit CD+4 T-cell responses to viral antigens. This was established by assessing the HCV-specific PBMC proliferative response in vitro, which depeneds on the presence of T cells that have been exposed to HCV antigens and have therefore undergone clonal expansion in vivo. However, the proliferative response intensity of PBMC is less than that of other’s report[3~6]. These are possible correlated with lower titer of HCV in infected body, inferior HCV immunogen, weak immune responses to HCV antigens in infec-ted host and not much purity of HCV antigens used in our experiment. From the point of view of selecting HCV predominant antigens core antigens (recombinant HCV C protein and synthetic peptide CP9) were a good immunogen because the PBMC proliferative responses to core antigens were the most vigorous in comparison with PBMC proliferative responses stimulated with any other HCV antigens (recombinant HCV E1,E2, NS3 and synthetic peptide NS4). This investigation result can provide a feasible method from cellular immune prevention for selecting HCV predominant antigens used in development HCV vaccine in future.
The phenotypic characteristics of proliferating cells indicated that HCV-specific CD+4 T lymphocytes proliferated more vigorous than that of CD+8 T lymphocytes measured by FACS. The result can suggest the PBMC stimulated with soluble exogenous antigens is expected to induce a preferential activation of CD4-positive HLA class Ⅱ-restricted T cells and not of CD8-positive HLA class Ⅰ-res-tricted cytotoxic T lymphocytes which are believed to be the main effectors for the elimination of virus-infected cells[11,12]. Because exogenous antigens are phagocytized and dealed with by macrophange and present antigens to the HLA-calss Ⅱ molecules to form heteromolecular complexes that are recognized by CD+4 T lymphocytes and induce CD+4 T lymphocytes to expand and proliferate.w, 百拇医药
The relationship between the immune res-ponse of PBMC from patients with hepatitis C to HCV antigens and outcome of infection is now difficult to determine. There are a few different opinions in some researchers([3~7]. Our study showed that the efficient proliferation response (SI2.1) of PBMC from patients with acute hepatitis C likely indicated that the lymphoproliferative response to HCV antigens are associated with clinical prognosis of HCV infection. Indeed, in the patients with acute hepatitis C, whose PBMC proliferative response to HCV antigens are stronger, their serum HCV-RNA were negative and serum ALT were normal when studied. In the other patients with acute and chronic hepatitis C, whose PBMC did not demonstrate efficient proliferative responses to HCV antigens, their serum HCV-RNA were detectable and serum ALT were elevated. Because the cases studied is not enough further studies in a large group of patients with hepatitis C and using more various antigens in PBMC proliferation assay are required. The present findings may show that PBMC from patients with hepatitis C exist proliferative responses to HCV proteins/peptides, of which core antigens were a good immunogen than any other HCV antigens used in our studies and suggest that the significant lympho-proliferative responses to HCV antigens are more frequently observed in acute infective patients who were able to eradicate the virus than in chronic HCV infective patients.
*This study was supported by the science foundation of public healih bureau of Zhejiang province in China.$5+]61f, 百拇医药
作者单位:Dou Jun(窦骏) Liu Kezhou(刘克洲) Chen Zhi(陈智) Wo Jian’er(沃建尔)He Nanxiang(何南祥) Xu Chenhuai(徐陈槐) Zhang Mingtai(章明太) Wang Xinzi(王信子) Institue of Infectius Disease ,Zhejiang Medical University ,Hangzhou 310003(Dou Jun now at:Department of Microbiology Nanjing Railway Medical College,Nanjing 210009)$5+]61f, 百拇医药
第一作者:男,43岁,博士,副教授$5+]61f, 百拇医药
REFERENCES$5+]61f, 百拇医药
[1] Dienstang JL. Hepatitis non-A, non-B:C at last. Gastroenterology, 1990,99:1177$5+]61f, 百拇医药
[2] Aler MJ,Margolis HS, Krawczynski K, et al. The natural history of community-acquired hepatitis C in the United statas. N Engl J Med, 1992,327:1899$5+]61f, 百拇医药
[3] Lechmann M, Ihlenfeldt HG, Braunschweiger I, et al. T-and B-cell responses to different hepatitis C virus antigens in patients with chronic hepatitis C infection and in healthy anti-hepatitis C virus-positive blood donors without viremia. Hepatology, 1996,24:790$5+]61f, 百拇医药
[4] Schupper H,Hayashi P,Scheffel J, et al. Peripheral-blood mononuclear cell responses to recombinant hepa-titis C virus antigens in patients with chronic hepatitis C. Hepatology, 1993,18:1055
[5] Botarelli P,Brunetto MR, Minutello MA, et al. T-lymphocyte response to hepatitis C virus in different clinical courses of infection.Gastroenterology, 1993,104:5082, http://www.100md.com
[6] Ferrari C,Valli A, Galati L, et al. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology, 1994,19:2862, http://www.100md.com
[7] Geert LR, Esquivel CA,Deleys R, et al. Lymphoproliferative responses to hepatitis C virus Core, E1,E2,and E3 in patients with chronic hepatitis C infection treated with interferon alfa. Hepatology, 1996,23:82, http://www.100md.com
[8] Kozied MJ,Dudley D,Afdhal N, et al. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C in persons with chronic hepatitis. J Immunol, 1992,149:33392, http://www.100md.com
[9] Han JH, shyanala V, Richman KH, et al. Characterization of terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5’ untranslated region and poly (A) tails at the 3’ end. Proc Natl Acad Sci USA,1991,88:17112, http://www.100md.com
[10] Chomczynski P, Sacchi N.Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem, 1987,162:1562, http://www.100md.com
[11] Kita H,Moriyama T, Kaneko T, et al. HLAB44-res-tricted cytotoxic T lymphocytes recognizing an epitope on hepatitis C virus nucleocasid protein. Hepatology, 1993, 18:10392, http://www.100md.com
[12] Kita H,Moriyama T,Kaneko T, et al. A Helper T-cell antigen enhances generation of hepatitis C virus-specific cytotoxic T lymphocytes in Vitro. T Med Virol, 1995, 45:3862, http://www.100md.com
(1998-04-03收稿;1998-07-13修回)(Dou Jun(窦骏), Liu Kezhou(刘克洲),Chen Zhi(陈智)1, Wo Jian’er(沃建尔),He Nanxiang(何南祥))